User:QuackGuru/Sand 9
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6795920/
https://en.wikipedia.org/w/index.php?title=Heated_tobacco_product
https://pubmed.ncbi.nlm.nih.gov/?linkname=pubmed_pubmed_citedin&from_uid=29535257&page=3
Highly toxic formaldehyde cyanohydrin is found in heated tobacco product aerosol.[2] Almost three times higher levels of potentially carcinogenic acenaphthene in heated tobacco product aerosol than in traditional combustible cigarette smoke have also been reported.[2]
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9021536/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10664078/ 10.1093/ntr/ntad177
https://www.euro.who.int/__data/assets/pdf_file/0008/443663/Heated-tobacco-products-brief-eng.pdf [4]
https://pubmed.ncbi.nlm.nih.gov/?term=Heated+tobacco+product&filter=years.2020-2021&size=200
https://pubmed.ncbi.nlm.nih.gov/?term=Heat-not-burn+product&filter=years.2019-2021&size=200



Heated tobacco products and heat-not-burn products generally heat dry tobacco[note 1][5] using a battery-powered heating system.[7] While heating, it generates an aerosol[7] and smoke[8] that contains nicotine,[7] gases, liquid and solid particles, tar,[8] and other chemicals, that is inhaled.[7] Prior to 2016, researchers at Philip Morris International stated that their IQOS product produces smoke[8] and the chemical evidence shows that the IQOS emissions fit the definition of both an aerosol and smoke.[9] The toxicity of IQOS increases each time it is not cleaned following use.[10] Heated tobacco products also contain additives not found in tobacco, and are frequently flavored.[7] They heat tobacco leaves to about 250–350 °C (around 500 °F[11])[12] or some as high as 550 ºC,[4] at a lower temperature than traditional cigarettes (600° C).[7] These products simulate the behavior of smoking traditional cigarettes.[7] To deliver nicotine from tobacco leaf, heated tobacco products use an embedded or external heat source, or a heated sealed chamber.[13] Some use product-specific customized cigarettes.[7] Some heated tobacco products have a similar size and shape as regular cigarettes and have a carbon tip wrapped in glass fibers that the user heats with a lighter or match.[6] There are devices that employ an induction heating system.[14] Although heated tobacco products are not e-cigarettes,[note 2][7] hybrids of heated tobacco products and e-cigarettes can make use of both tobacco and e-liquid.[13] There are a various types of heated tobacco products and heat-not-burn products,[5] as well as devices that use cannabis.[6]
Heated tobacco products are not without risk[15] and their long-term effects are unknown.[16] A 2016 World Health Organization report found no compelling independent research to support claims of lowered risk or health benefits compared with traditional cigarettes.[17] There is insufficient evidence to conclude that heated tobacco products are less harmful than conventional cigarettes.[18] The research indicates that heated tobacco products are more hazardous than e-cigarettes.[19] The aerosol contains levels of nicotine and carcinogens comparable to regular cigarettes.[20] While animal studies, and human clinical studies by Philip Morris International researchers claim that IQOS aerosol is significantly less harmful to human health than conventional cigarette smoke, findings from independent reviews of Philip Morris International's own data shows that IQOS aerosol is as harmful as conventual cigarette smoke to human health.[9] Non-users can be exposed to toxic chemicals.[16] The limited evidence on air emissions from the use of heated tobacco products indicates that toxic exposure from these products is greater than that of e-cigarettes.[21]
The nicotine in these products make them highly addictive.[22] The nicotine content between heated tobacco product and traditional cigarette emissions are in similar ranges, which suggests a similar addictiveness and dependence potential.[12] Heated tobacco products causes cancer in humans.[23] Several studies demonstrate nicotine is carcinogenic.[24] There is insufficient evidence on the efficacy of heated tobacco products on quitting smoking.[25] Dual use of heated tobacco product regular users with combustible smoking products is common.[26] Trying a heated tobacco product was more frequent among adults below the age of 30 and regular traditional cigarette users.[27] The risk to the fetus from heated tobacco products during pregnancy is hard to quantify, though the risk to the fetus is probably less than traditional smoking during pregnancy.[28] Nicotine is harmful to the infant and the growing adolescent brain.[20] Nicotine is especially harmful to children and adolescents.[16] Using nicotine in adolescence may increase the risk for future addiction to other drugs.[6]
As early as the 1960s, tobacco companies developed alternative tobacco products intended to supplement the cigarette market.[29] Heated tobacco products entered the market in 1988; though they were not a commercial success.[11] There has been a global decline in tobacco consumption that, if continued, will negatively impact the tobacco industry's profits.[29] This decline led the industry to invent and market new products like heated tobacco products.[29] Smokers have reported heated tobacco product use to be less satisfying than smoking a cigarette.[21] In 2016, the latest generation of heated tobacco products appeared to be the most recent industry attempt to partner with governments and health advocates, presenting claimed 'harm reduction' products as an option to address the tobacco epidemic.[29] The tobacco industry promotes heated tobacco products as a safer alternative to traditional cigarettes with misleading marketing sustained by studies with a conflict of interest.[30] Current smoking bans may or may not apply to heated tobacco products.[31] Heated tobacco products contribute to the overall stream of electronic waste.[32]
Health effects
Limited research

A 2016 Cochrane review found it unclear if the use of heated tobacco products would "substantially alter the risk of harm" over traditional cigarettes.[33] As of December 2017[update], it is impossible to quantify the health risk from using these products.[28] There is very limited information available on health effects[28] and the available research on their harmfulness is limited.[34] The short-term adverse effects[35] as well as the long-term effects are unclear.[35] There is a lack of long-term studies.[12] As of 2019[update], a limited amount of independent studies have been conducted on heated tobacco products.[36] The different types of available heated tobacco products vary in features and effect, creating a challenge for researchers.[37]
Safety
There is insufficient evidence to conclude that heated tobacco products are less harmful than conventional cigarettes.[18] Heated tobacco products are not harmless to health.[15] Research has shown that the benefits of heated tobacco products are controversial.[18] The use of any tobacco product—including heated tobacco products—is harmful, especially for youth, young adults, and pregnant women, as well as adults who do not currently use tobacco products.[6] A 2016 World Health Organization report stated that claims of lowered risk or health benefits for heated tobacco products compared with traditional cigarettes were based on industry-funded research, and compelling independent research was not available to support these claims.[17] Studies on human use were wide-ranging and largely affiliated with the manufacturers, as of 2018.[38] Action on Smoking and Health in the UK stated in 2016 that due to "the tobacco industry's long record of deceit" regarding the health risks involving smoking, it is important to conduct independent studies into the health effects of these products.[39]
With an assorted range of electronic cigarettes devices available in the UK, it is unclear whether heated tobacco products will offer any favorable benefit as a plausible harm reduction product.[19] In 2016, a World Health Organization report noted that some scientists believe that heated tobacco products were as dangerous as traditional cigarettes.[17] In 2017, the Committee on Toxicity states that heated tobacco products may be less dangerous than smoking, but it recommends it would be better for smokers to completely stop.[28] The current evidence indicates that heated tobacco products are less safe than e-cigarettes.[19]
Emissions
Heated tobacco products are frequently flavored and contain additives not found in tobacco.[7] Some harmful compounds can be decreased during heated tobacco product use, though heated tobacco product users can inhale higher amounts of other harmful compounds in contrast to tobacco smoke.[40] The aerosol contains levels of nicotine and carcinogenss comparable to regular cigarettes.[20] They contain comparable levels of many volatile organic compounds and greater amounts of the polycyclic aromatic hydrocarbon acenaphthene than regular cigarettes.[41] Some of the chemical substances detected in heated tobacco products are listed in Agents Classified by the International Agency for Research on Cancer Monographs as carcinogenic to humans (group 1) (formaldehyde), probably carcinogenic to humans (group 2A) (acrolein), and possibly carcinogenic to humans (group 2B) (acetaldehyde and lead).[42] The substances in the emissions of traditional cigarettes such as tar, nicotine, carbonyl compounds (including acetaldehyde, acrolein, and formaldehyde), and nitrosamines are also found in emissions of heated tobacco products.[43] A 2017 study found a 10% rise in carbon monoxide and formaldehyde air levels than compared to the background during heated tobacco product use.[44] A 2017 study found heated tobacco products generated emissions of metal particulates, organic compounds, and aldehydes, and research suggests that heated tobacco products generate less concentrations of airborne contaminants in indoor places in comparison to a traditional cigarette,[44] though their use still reduces indoor air quality.[36] Over 20 toxic and potentially toxic chemicals are substantially greater in heated tobacco emissions than in cigarette smoke.[45] Temperature alters the composition of the emissions.[8] Their long-term effects from heated tobacco emissions exposure is unknown.[16] Heated tobacco products produce emissions that are not as safe as clean air.[6]
A 2018 Public Health England report found that "Compared with cigarettes, heated tobacco products are likely to expose users and bystanders to lower levels of particulate matter and harmful and potentially harmful compounds (HPHC). The extent of the reduction found varies between studies."[37] They also noted that the evidence indicates that less nicotine was inhaled from heated tobacco products than cigarette smoke.[46] Exposure to mutagenic and other harmful substances is lower than with traditional cigarettes, though reduced exposure to harmful substances does not correlate with reduced health risk severity.[12] Even low exposure increases the risks for cancers, stroke, and other cardiovascular diseases compared to non-smokers.[12] To what extent the reduced levels result in lowered health risks remains unclear.[12] Lower levels of harmful emissions have been shown, but lowering the risk to the smoker who transitions to using them has not been shown, as of 2018.[8] In 2017, the Committee on Toxicity found that heated tobacco products do not reduce exposure or potential addiction to nicotine; some of the substances inhaled from using these products are carcinogens.[28]
Physiological changes in response to heated tobacco emissions, such as multiple organ system inflammation, energy metabolism, and carcinogenesis, have not been well characterized due to limited research in this area, especially in animal models.[25] A 2018 in vitro study suggested a less harmful pathophysiological response in human organotypic oral epithelial cultures when exposed to such emissions.[25] A 2016 animal study showed that heated tobacco emissions did not increase surfactant lipids and proteins, inflammatory eicosanoids and their metabolic enzymes, and several ceramide classes in heated tobacco product-exposed mice when compared with their counterparts that were exposed to cigarette smoke.[25] It also discovered that even with reduced toxicants in heated tobacco product emissions, overuse (40 tobacco sticks per day) can still lead to eosinophilic pneumonia in humans.[25]
The impact on the overall population is unclear.[35] Non-users can be exposed to toxic chemicals.[16] Studies on second-hand heated tobacco product emissions as of 2018 were diverse and largely affiliated with manufacturers.[38] There is disagreement over the extent to which heated tobacco products generate air emissions, and the emissions' composition.[47] There is anticipated to be a reduced risk to bystanders where smokers were using heated tobacco products instead of smoking.[28] Limited evidence on air emissions indicates that toxic exposure from heated tobacco products is greater than from e-cigarettes.[21] Levels of tobacco-specific nitrosamines in IQOS, which are carcinogenic, were substantially greater than e-cigarettes.[48] Heated tobacco products has been shown to emit more polycyclic aromatic hydrocarbons and carbonyl compounds than is observed in e-cigarette fumes.[18]
Carcinogenicity
Heated tobacco product emissions generate carcinogens and potential carcinogens[23] and are more danger than vaping in terms of risk of getting cancer.[49] Nicotine poses an array of health risks[24] such as the stimulation of cancer development and growth.[50] The International Agency for Research on Cancer does not consider nicotine to be a carcinogen, though several studies demonstrate it is carcinogenic.[24] Because it can form nitrosamine compounds (particularly N-Nitrosonornicotine (NNN) and nicotine-derived nitrosamine ketone (NNK)) through a conversion process, nicotine itself exhibits a strong potential for causing cancer.[51] About 10% of breathed in nicotine is estimated to convert to these nitrosamine compounds.[51] Nitrosamine carcinogenicity is thought to be a result of enhanced DNA methylation and may lead to an agonist response on the nicotinic acetylcholine receptors, which acts to encourage tumors to grow, stay alive, and penetrate into neighboring tissues.[51]
Addiction and quitting
Heated tobacco products contain the highly addictive chemical nicotine.[22] The nicotine content between heated tobacco product and traditional cigarette emissions are in similar ranges, which suggests a similar addictiveness and dependence potential.[12] There is insufficient evidence on the efficacy of heated tobacco products on quitting smoking.[25] Heated tobacco products do not provide assistance to smokers to stop using tobacco altogether.[16] The usefulness of heated tobacco products for quitting smoking remains controversial[52] and uncertain.[53]
In 2016 Philip Morris International acknowledged that IQOS is probably as addictive as tobacco smoking.[39] IQOS is sold with a warning that states the best option is to avoid tobacco use altogether.[54] IQOS can record the users smoking habits, while Philip Morris International stated it only retrieves the personal data when the product is not working properly.[55] Gregory Connolly, a professor at Northeastern University who has studied IQOS, said in 2019 that Philip Morris International will have a "mega database" of Americans' smoking habits, and then possibly "reprogram the current puffing delivery pattern of the iQOS to one that may be more reinforcing and with a higher addiction potential".[55]
Nicotine yield
The limited data on heated tobacco product users show that they take short puffs and that the time between puffs is very short.[8] Experimental tests show a higher volume of puffs at shorter intervals than with traditional cigarettes.[8] A 2018 clinical trial found that tests of smokers switching to IQOS showed a tendency to take more puffs at shorter intervals.[8]
Evidence indicates that the concentrations of nicotine in mainstream heated tobacco product aerosols are lower than in cigarette smoke.[46] Tested heated tobacco products provided more nicotine in the aerosol than a cigalike e-cigarette but not as much nicotine compared with a tank-style e-cigarette.[21] Smokers regularly reported heated tobacco product use to be less satisfying than smoking a cigarette.[21]
Users experience blood nicotine levels that peak after about six to seven minutes for both heated tobacco products and traditional cigarettes.[8] The IQOS produces slightly less blood nicotine overall than a traditional cigarette, but more than nicotine gum.[8] A 2016 study of 160 smoking trial volunteers found that switching to IQOS, after an initial adjustment period, usually smoked more traditional cigarettes than those not switching, while reporting that they were less satisfying, less rewarding, and had a lower reduction in cravings than with traditional cigarettes.[8] The study noted that more than 30% of the volunteers had headaches, irrespective of what was used.[8]
Sharper peaks in blood nicotine levels from inhalation cause greater nicotine dependence than oral consumption.[56] Nicotine replacement products, for instance, deliver nicotine in a slow, stable manner, which is less addictive.[56] Inhaled nicotine enters the blood quicker than oral consumption, and blood nicotine levels halve every one to two hours.[56] Nicotine withdrawal causes deteriorating mood and creates a craving for nicotine consumption.[56]
Vascular disease
The detrimental effects of aerosols associated with heated tobacco products start at the endothelial level.[57] The oxidative stress reduces the bioavailability of nitric oxide which in turn plays a key role in endothelium dysfunction alongside peroxynitrite (produced by superoxide) which causes low-density lipoprotein oxidation and inflammatory cytokines.[57] A healthy endothelium is responsible for the production of vasodilators; however this function becomes impaired when the endothelium is injured (e.g., by cigarette smoke or toxic compounds found in heated tobacco products) leading to an imbalance between vasoconstrictors and vasodilators.[57] The damaged cells from the endothelium must be replaced in order to maintain vascular tone, an ability that is reduced in users of heated tobacco products.[57] Platelet activation, coagulation cascade stimulation, and impairment of anticoagulative fibrinolysis leads to vascular disease.[57] IQOS emissions damages the vascular endothelia function of rats comparible to those inhaling cigarette smoke.[48]
Respiratory effects
Heated tobacco product use is involved in damage related to the development of lung diseases.[30] Heated tobacco products can result in serious lung injury and shortness of breath which can lead to death following a short period of use.[58] A case report of acute eosinophilic pneumonia after using a heated tobacco product was published in the literature in 2016.[59]
Studies assessing the biomarkers of exposure to heated tobacco products found a reduction of exposure in participants switching to heated tobacco products from conventional cigarettes, which may suggest a decreased risk of developing smoking-related diseases.[18] On the other hand, exposure to IQOS has been reported to alter mitochondrial function, which may further exaggerate airway inflammation, airway remodeling and lung cancer.[18] These products have the potential to increase oxidative stress and increase respiratory tract infections by increasing microbial adherence to the respiratory tract.[18]
Cardiovascular effects
Both in vivo and in vitro studies have shown that this type of cigarette exhibits a deleterious effect on the cardiovascular system.[57] The amounts of nicotine, particulate matter, benzene, acrolein, and tobacco-specific nitrosamines emissions are less in contrast to traditional cigarettes, but are possibility detrimental to cardiovascular health.[48] Heated tobacco products create tiny particles that can penetrate the lung and they may harm lung tissue.[60]
Pregnancy
Pregnant women who wish to quit smoking but are unable to are left with few options.[61] As nicotine replacement products are often ineffective for quitting smoking, pregnant women turn to alternatives such as heated tobacco products.[25] There is no information available on the potential impact of heated tobacco product emissions from mother to fetus as of 2018[update].[25] The risk to the fetus from heated tobacco products during pregnancy is hard to quantify.[28] Although the risk to the fetus is probably less than traditional smoking during pregnancy, the Committee on Toxicity recommends that expectant mothers completely stop smoking.[28] Nicotine is harmful to the infant[20] is metabolized much faster while a woman is pregnant, easily passes through the placental barrier, and collects in breast milk.[56] There is also growing evidence that nicotine exposure during pregnancy is linked to early birth, stillbirth, and abnormal brain growth.[56] Nicotine is harmful to developing fetuses.[6] Nicotine may result in adverse effects to the neurological growth of the fetus.[62]
Nicotine can lead to vasoconstriction of uteroplacental vessels, which reduces the delivery of both nutrients and oxygen to the fetus.[25] As a result, nutrition is re-distributed to prioritize vital organs, such as the heart and the brain, at the cost of less vital organs, such as the liver, kidneys, adrenal glands, and pancreas, which can lead to underdevelopment and functional disorders later in life.[25] Animal research in regards to maternal nicotine exposure on rats showed a direct adverse impact on pancreas development by reducing endocrine pancreatic islet size and number, which was accompanied by a decrease in gene expression of specific transcription factors and blood glucose regulating hormones such as insulin and glucagon.[25] Affected rats exhibited significant pancreatic dysfunction and glucose intolerance.[25] Other animal studies have reported insulin resistance in adult offspring due to maternal nicotine exposure; in animal models, nicotine has also been shown to activate nicotinic acetylcholine receptors (nAChRs) in the brain, which regulate brain development.[25] Nicotine exposure during the first trimester of pregnancy (2 mg/kg/d) leads to structural changes in the hippocampus and somatosensory cortex in rats.[25]
Youth
Nicotine is especially harmful to children and adolescents.[16] Nicotine exposure can harm the developing adolescent brain.[6] The brain keeps developing until about age 25.[6] Using nicotine in adolescence can harm the parts of the brain that control attention, learning, mood, and impulse control.[6] Using nicotine in adolescence may also increase the risk for future addiction to other drugs.[6]
Public perception
It is unknown how users evaluate product safety.[63] A survey showed that among those who have tried heated tobacco products, approximately 50% believe they are safer than traditional cigarettes, while the other 50% believe they are just as unsafe as traditional cigarettes.[63] Need copy.
Public health implications
Their use is a public health calamity.[64] Significant controversy is associated with their impact on public health.[65] The public health impact of heated tobacco products depends not only on whether they are less harmful than traditional cigarettes, but whether they encourage an increase or decrease in the prevalence of smoking.[65]
Tooth discoloration
There was low‐quality evidence to suggest that novel tobacco and nicotine products such as heated tobacco products cause staining of dental hard tissues and dental materials; this was present to a lower extent than with cigarette smoke.[66]
Usage
Prevalence
There is minimal data available regarding product use, including whether smokers will tend to abandon tobacco smoking entirely or continue using both kinds of products.[67] Additionally, initiation by people who have never smoked, including young individuals, remains an area lacking comprehensive data.[67] The impact of heated tobacco products on influencing non-smokers to start smoking remains uncertain.[68]
In 2017, the Committee on Toxicity stated, "The Committees were concerned over the potential for non-smokers including children and young people, who would not otherwise start to smoke cigarettes, to take up using these products as they are not without risk."[69] They also stated that the "Committees were particularly concerned for young people, who do not smoke, starting to use these products, due to the potential for longer exposure over the remainder of their lives compared to adults and to possible differences in sensitivity."[69]
Dual use of heated tobacco product regular users with combustible smoking products is common.[26] Trying a heated tobacco product was more common among adults below the age of 30 than people over the age of 30, and was more common among regular traditional cigarette users than people who had never smoked.[27] A 2015 online survey found that 6.6% of 8240 respondents had tried a heated tobacco product at least once.[70] Research demonstrated that there is a high overlap between heated tobacco product users and women who smoke.[63] Previous smokers with less education have a somewhat higher tendency to ever use heated tobacco products.[40]
International
Between 2015 and 2020, heated tobacco product use went up in Europe and the Western Pacific, with close to 5% of the people having experimented with a heated tobacco product.[71] 1.5% in these regions describe themselves as current users.[71]
International youth
In the US, Canada, and England, tendency of youth to experimenting with IQOS was a bit less than e-cigarettes, but greater than cigarette use. [48]
Germany
In Germany, heated tobacco product use is not common and is generally more frequent among richer and more educated smokers.[63]
Italy
Surveys found that 96% of Korean IQOS users are also cigarette smokers, and 45% of Italian IQOS users had not tried smoking cigarettes.[48] In Italy, heated tobacco product use was 1.4% among the people and 3.1% of the heated tobacco product users were regular tobacco users.[63]
Japan
Since its sale in Japan in 2014, IQOS use has increased.[63] A 2017 survey in Japan found that of those who used the IQOS within the last 30 days, 20% had never smoked before.[8] In Japan in 2017, heated tobacco products did not satisfy 86% of users, and they did not quit using traditional cigarettes; they used both.[8]
United States
As of July 2017, not many US adults had tried using a heated tobacco product.[27] As of July 2017, approximately one in twenty US adults (including one in ten traditional cigarette users) have heard of heated tobacco products.[27] In 2018, few US adults (2.4% of all surveyed, including 6.7% of people who currently smoke surveyed) had ever used heated tobacco products.[72]
Although heated tobacco products are new to the market as of 2020, they are gaining in popularity.[73] Just six months after the US FDA allowed the sale of heated tobacco products, 18% of California adults were aware of them and 4% reported having tried heated tobacco.[73] 2% of California adults report currently using heated tobacco.[73] Research from Japan, where heated tobacco products have been available since 2013, show that nearly 70% of adult heated tobacco users also smoke cigarettes.[73] To date, there is little information about heated tobacco product use among youth.[73]
United States youth
In 2020, 1.4% of US middle and high school students, combined, reported having used heated tobacco products in the past 30 days.[72] In 2022, less than one of every 100 middle school students (0.7%) and about 1 of every 100 high school students (1.1%) reported using heated tobacco products in the past 30 days in the US.[74]
Motivation
The availability of flavors in heated tobacco products may appeal to non-smokers,[34] and a growing body of evidence shows that individuals who have never used tobacco products, especially children and adolescents, could be susceptible to new products that could lead to the use of traditional cigarettes.[20] IQOS packaging could be enticing to youth.[75] IQOS is being promoted as a high-tech, prestigious product.[75] Since youth and young adults are intrigued in such things, this technique heightens concerns regarding youth enticement.[75]
Gateway theory
A 2018 World Health Organization report states that "Conclusions cannot yet be drawn about their ability to assist with quitting smoking (cessation), their potential to attract new youth tobacco users (gateway effect), or the interaction in dual use with other conventional tobacco products and e-cigarettes."[7] A 2018 survey in Italy found that 45% of people who experimented with the IQOS and 51% who were interested in the product had never smoked before.[8] Therefore, such a product may represent, at least in Italy, a gateway for nicotine addiction among people who had never smoked before rather than a harm reduction substitution for current smokers.[61]
According to four epidemiological papers, 10 to 45% of non-smokers use these products and this research shows the effectiveness of the marketing of the tobacco industry.[8] With that said, the heated tobacco product known as IQOS acts more as a gateway to traditional cigarette use (20% of users) than as a means of quitting (11% of users), and it is not anticipated to have a lowered health risk among dual users who make up the remaining 69%.[8] Philip Morris has previously stated that 8.8 million people have quit using traditional cigarettes in place of IQOS; but, evidence indicates that it may serve as a gateway or an addition to cigarette use, instead of a substitute.[48]
Construction

Nicotine is released from tobacco heated above 150 °C.[76] Combustible tobacco cigarettes reach about 900 °C during a puff and smoulder at about 400 °C between puffs.[77] The burning process, substances emitted and their levels vary at different temperatures: distillation—the process during which nicotine and aromas are transferred from tobacco to smoke—occurs below 300 °C; pyrolysis occurs around 300 °C–700 °C and involves the decomposition of biopolymers, proteins, and other organic materials and generates the majority of substances emitted in smoke; and combustion occurs above 750 °C and results in the generation of carbon dioxide, carbon monoxide, and water.[77]
The temperature the tobacco reaches greatly varies among heated tobacco products; it depends on the process used to heat the tobacco.[28] For example, HeatSticks are heated to a maximum of 350 °C, a temperature sufficient to enable pyrolytic decomposition of some organic materials,[77] while the glo iFuse heats tobacco to around 35 °C.[8] The formation of toxic volatile organic compounds, including formaldehyde, acetaldehyde, and acrolein have been reported in e-cigarette aerosols at similar temperatures as the IQOS.[77] Carbon monoxide (CO), nitrogen oxides, soot or tars, and aldehydes in the emissions demonstrate that heated tobacco products undergo thermal degradation.[8] Gases, liquid, and solid particles are also found in the emissions; the solids in the emissions have been called nicotine-free dry particulate matter rather than tar in papers written by people connected to the tobacco industry.[8]

Since the constituents of HeatSticks may differ from combustible cigarettes, including flavorants and additives, it is plausible that the IQOS aerosol may contain substances not found in tobacco smoke.[77] The emissions of the IQOS HeatSticks and the IQOS menthol mini-cigarettes contain about three times the amount of water and about half the amount of tar found in traditional cigarette emissions.[8] The IQOS HeatSticks do not generate a flame,[78] but are charred.[30] This charring increases when the product is not cleaned following the use of each heat stick.[30] Prior to 2016, researchers at Philip Morris International stated that their IQOS product produces smoke[8] and the chemical evidence shows that the IQOS emissions fit the definition of both an aerosol and smoke.[9]
The IQOS available in Germany consists of three components with different functions: the processed tobacco stick; a pen-like heater (holder) that the tobacco stick is inserted, which is then heated by an electrically controlled heating element; and a charger that recharges the heater after use.[12] The heated tobacco products automatically stops the heating process after six minutes or 14 puffs so that pyrolytic products and pollutant release are limited in time as well as by a maximum number of puffs per stick.[12] Heated tobacco products produce side-stream emissions.[4] Heated tobacco products reach temperatures that are sufficient for pyrolysis to happen.[43] There are devices that use a reaction that resembles pyrolysis or combustion, but research has not determined which of the two it is.[8]
The tobacco stick contains a compressed tobacco film made of a dried tobacco suspension that has been rolled up into a paper-thin brown tobacco foil and several filter elements.[12] This film consists of about 70% tobacco, as well as humectants (such as water and glycerin, to prevent the tobacco from drying out and promote aerosol formation), binders, and flavorings.[12] The filter elements consist of two independent systems: a polymer film filter that cools the aerosol, and a soft cellulose acetate mouthpiece filter that mimics the sensory aspects of a traditional cigarette.[12]

Heated tobacco products are battery-powered systems that produce nicotine-containing emissions by heating tobacco.[12] For this purpose, tobacco sticks are placed in a corresponding heater and heated to about 250–350 °C (around 500 °F[11])[12] or some as high as 550 ºC,[4], which result in nicotine-containing emissions, that are inhaled via a mouthpiece with a filter segment.[12] Heated tobacco products are hybrids between electronic and conventional cigarettes: they are equipped with a device that heats the product, without reaching combustion, to generate aerosol (i.e., a sort of "cold smoke"), while using "real" tobacco instead of nicotine-containing liquids.[61] There are products that have a time limit so that the user is forced to inhale the nicotine within 3.5 to 10 minutes prior to the device turning off.[8] This function helps support blood nicotine peaks that result in an increased nicotine dependence.[8]
There are three general types of heated tobacco products.[28] One that immediately heats processed tobacco to generate aerosol, another that uses processed tobacco that is heated in an aerosol, and one where the processed tobacco gives flavor to the aerosol as the latter moves over the former.[28] Heated tobacco products heat tobacco leaves at a lower temperature than traditional cigarettes.[25] Another type of heated tobacco product is the loose-leaf tobacco vaporizer that involves putting loose-leaf tobacco into a chamber that is electrically heated with an element.[79] Some use product-specific customized cigarettes.[7] Although heated tobacco products are not e-cigarettes,[7] hybrids of heated tobacco products and e-cigarettes can make use of both tobacco and e-liquid.[13]
History
Heated tobacco products were not a commercial success, and most of them were quickly taken off the market following their debut.[11] Heated tobacco products have been introduced by large tobacco companies.[80] They have been developed by several tobacco companies.[81]
As early as the 1960s, the tobacco companies developed alternative tobacco products with the intent to supplemet the cigarette market.[29] The first commercial heated product was the Premier by R. J. Reynolds,[82] a reportedly smokeless cigarette launched in 1988 and described as difficult to use.[83] Many smokers disliked the taste,[84] and it was not popular with users when it was test-marketed in Arizona and Missouri.[85] It was shaped like a traditional cigarette[86] and required some combustion.[87] While heated the smoldered charcoal moved past processed tobacco containing more than 50 percent glycerin to create a nicotine-containing aerosol.[86] In 1989,[88] after spending $325 million,[89] R. J. Reynolds pulled the Premier from the market months later after the American Medical Association and other organizations recommended that the US Food and Drug Administration (FDA) restrict it or classify it as a drug.[90]
The Premier product concept was developed further and re-launched as the Eclipse[88] in the mid-1990s,[91] which was available in limited distribution as of 2015[update],[92] and promoted via viral marketing.[88] The emissions from earlier generation of heated tobacco products (e.g., Eclipse) were classed as smoke by a 2004 study affiliated to the manufacturer, and the emissions were also found to contain soot (black carbon).[9] Reynolds American also introduced a brand called Revo and stated that it was a "repositioning" of the Eclipse.[93] The Revo was withdrawn in 2015.[92]
The Steam Hot One was sold in Japan by Japan Tobacco.[94]
In October 1998, Philip Morris launched the Accord in the US.[95] It was a specialized cigarette which was designed to be used with the electric heating system.[95] Advertisements stating reduced risk were drafted for the Accord in the US, but were never released.[95] In 1998, the company launched the Accord in Osaka, Japan, and renamed it Oasis.[95] The battery-powered, pager-size product[96] was marketed as "low-smoke".[95] An attempt was made in 2007 by Kenneth Podraza, the Vice President of Research and Development at Philip Morris in the US at the time, to get the Surgeon General of the United States to endorse it.[95] The Surgeon General did not reply to Podraza's letter.[95] Few people used the Accord, and most of them also continued to use traditional cigarettes.[95] The Accord ceased production in 2006, eight years after it came on the market.[95]
In 2007 Philip Morris International launched the Heatbar,[97] which was very similar to the Accord.[95] It was around the size of a mobile phone and was said to heat specially-designed cigarettes rather than burning them.[98] The Heatbar did not obtain any significant user reception,[99] and was discontinued after the only benefit found was to lower second-hand smoke.[100] The Accord and Heatbar are predecessors of Philip Morris International's current heated tobacco products.[101]
In years leading up to 2018, increased tobacco control measures have directed the tobacco industry to develop alternative tobacco products, such as heated tobacco products.[12] There has been a global decline in tobacco consumption that, if continued, will negatively impact the tobacco industry's profits, which has forced the industry to invent and market new products like heated tobacco products.[29] The introduction of heated tobacco products may also have been a response to the growing popularity of e-cigarettes beginning around 2007 after independent companies introduced them before major multinational tobacco companies entered the e-cigarettes market.[29]
The global decline of cigarette consumption and decrease in adult smoking prevalence (from 24% in 2007 to 21% in 2015), combined with the success of tobacco control, including the implementation of the WHO Framework Convention on Tobacco Control, may also have led the tobacco companies to consider alternative products to protect their profits and political interests.[29] T.L. Caputi suggests that the ubiquity of e-cigarettes and the growing dissatisfaction with not providing a "throat-hit"[note 3] may present an opportunity for heated tobacco products.[11] Philip Morris International anticipates a future without traditional cigarettes, but campaigners and industry analysts question the probability of traditional cigarettes being overshadowed by either e-cigarettes or other products like the IQOS.[103]
Products

Heated tobacco products use a heating system where the tobacco is heated and aerosolized.[7] Heated tobacco products are designed to be similar to their combustible counterparts[104] and they simulate the behavior of smoking traditional cigarettes.[7] They replicate the oral inhalation and exhalation, taste, rapid systemic delivery of nicotine, hand-to-mouth feel and throat hit sensations (depending on the temperature) that are like smoking traditional cigarettes.[25] Heated tobacco products aim for a niche between combustible tobacco smoking and e-cigarettes that aerosolize nicotine.[38] There are different types of heated tobacco products and heat-not-burn products in the marketplace;[5] some use tobacco sticks like glo and IQOS, while others use loose-leaf tobacco such as Pax and Ploom.[7]
A 2020 World Health Organization report stated that later models of heated tobacco products "include lower- and higher-temperature variants, hybrid electronic devices with both tobacco and liquid, carbon-tipped devices, devices using a metallic mesh punctured with tiny holes to heat a pre-filled, pre-sealed liquid cap, and others which allow users to customize the temperature and manage the aerosol and flavour output."[16] Some heated tobacco products use electronic heating elements.[6] Some heat specially-designed sticks, plugs, or capsules containing tobacco.[6] Some work by heating liquids that create an emission that then passes through a tobacco plug to absorb flavor and nicotine from the tobacco.[6] Some have a sealed part of the device that heats loose tobacco, either alone or together with flowers from the marijuana (cannabis) plant.[6] Some heated tobacco products have a similar size and shape as regular cigarettes and have a carbon tip wrapped in glass fibers that the user heats with a lighter or match.[6]
Cigoo
In September 2020, Yunnan Xike Science & Technology Co., Ltd. launched Cigoo;[105] according to the company, it is a heated herbal product which releases nicotine and aroma aerosol at 3000 °C, similar to mainstream heated tobacco products.[105] Instead of using reconstituted tobacco film in the stick,[12] Cigoo sticks use patented plant particle as a carrier, added flavorants and additives.[106]
Firefly vaporizers
The Firefly developed the Firefly 2, which heats loose-leaf plant material and concentrates and is often used to aerosolize cannabis,[107] and is more compact than the original Firefly vaporizer.[108] As with the Firefly 2, the Firefly 2 Plus uses a patented heating technology, which heats the device up to the desired temperature with each puff rather than a preset temperature setting from the beginning.[109]
glo products
In 2016, British American Tobacco launched a battery-powered heated product called glo in Japan.[110] It is also sold in South Korea, Switzerland, Russia,[111] and Ukraine.[112] In France, glo uses tobacco sticks called Neostiks.[8] It uses a heating element with a tobacco stick,[104] known as a mini-cigarette.[8] glo heats up to 240 °C.[8] glo produces approximately 50% less nicotine emissions than IQOS.[36] In May 2017 British American Tobacco released i-glo in Canada.[113] Bonnie Herzog, a senior analyst at Wells Fargo Securities stated that the proposed acquisition of R. J. Reynolds by British American Tobacco in 2016 would let them catch up technologically with the competition.[114] The data on glo is limited.[67] glo is marketed as being easier to operate than IQOS.[112]
The glo iFuse debuted in Romania in 2015,[104] and is a hybrid of a heated tobacco product and an e-cigarette.[77] It consists of a heating element, a liquid tank (like e-cigarettes), and a tobacco cavity through which the aerosol passes and is infused with tobacco flavor.[77] It uses cartridges called Neopods, and heats tobacco to approximately 35 °C.[8] This lower temeperature differs from other heated tobacco products.[8]
IQOS

IQOS (/ˈaɪkoʊs/ EYE-kohs[115]) was introdcued in June 2014[116] and marketed by Philip Morris International under the Marlboro and Parliament brands.[117] IQOS is not an acronym for "I Quit Ordinary Smoking," according to Philip Morris International's chief executive officer André Calantzopoulos,[118] though some have made this claim.[115] Although it is marketed as a novel product, it is very similar to the Accord released by the same company in 1998; however, the IQOS sticks have more nicotine, more tar, and less tobacco.[95] They are heated at a lower temperature than Accord.[95] Philip Morris International invested $3 billion and exceeded 1900 filed patents, connected to IQOS.[119]
Initially launched in 2014 in Nagoya, Japan, and Milan, Italy, IQOS has been introduced out to other countries.[120] As of November 2021[update], IQOS products are available in 69 countries.[121] Philip Morris International has projected that when 30 billions units are sold, IQOS would increase profits by $700 million.[122] In October 2018, Philip Morris International introduced a less expensive version of IQOS called IQOS 3 in Tokyo, Japan.[123] The IQOS 3 Multi was also launched, and is capable of multiple consecutive uses.[124] Debuted in Japan in August 2021, the IQOS Iluma as well as the IQOS Iluma Prime employ an induction heating system.[14]
The IQOS consists of a charger roughly the size of a mobile phone and a pen-like holder.[125] The IQOS packaging is similar to iPhones.[126] The disposable tobacco stick[127] also known as HeatSticks or HEETS in some places they are sold,[128] is described as a mini-cigarette.[8] The sticks contain processed tobacco soaked in propylene glycol.[127] The stick is inserted into the holder which then heats it to temperatures up to 350 °C,[129] and the amount of nicotine provided may be a little strong for light cigarette smokers.[130] IQOS sticks contain 70–80% of the concentration of nicotine found in traditional cigarettes.[18] The sticks are available in various flavors.[130] Users have reported less smell and odor on clothing.[87]
There is a limited amount of research on the effect of IQOS on the user's health.[131] A scant amount of research has been conducted regarding the substances that are emitted from the apparatus once it heats the tobacco-derived mixture.[132] Significant levels of n-alkanes, organic acids, and carcinogenic aldehydes including formaldehyde, acetaldehyde, and acrolein have been observed in IQOS side stream aerosol.[9] The emissions generated by IQOS contain the identical harmful constituents as tobacco cigarette smoke, including volatile organic compounds at comparable levels to cigarette smoke, polycyclic aromatic hydrocarbons at various ranges, and carbon monoxide.[20] Each of these substances, on the basis of rigorous research of cigarette smoke, are known to result in significant harms to health.[20]
Philip Morris International states that IQOS is less dangerous than traditional cigarettes, but this statement is unsubstantiated by their own research.[48] While animal studies, and human clinical studies by Philip Morris International researchers claim that IQOS aerosol is significantly less harmful to human health than conventional cigarette smoke, findings from independent reviews of Philip Morris International's own data shows that IQOS aerosol is as harmful as conventual cigarette smoke to human health.[9] Continual reheating of deposited tar in the IQOS device will occur with real-life use, likely leading to generation of even higher concentrations of harmful and potentially harmful compounds and particulate matter.[9]
People that replaced traditional cigarettes with IQOS had not improved in pulmonary function or inflammation, according to Philip Morris International's own pulmonary research.[48] IQOS use has a negative effect on airway function.[48] IQOS aerosols demonstrate cytotoxicity in bronchial epithelial cells of humans as well as other cells of the respiratory system, according to in vitro research.[48] The cytotoxicity of the IQOS was higher toxicity than that of e-cigarettes.[18] The data suggest that the use of IQOS products may lead to an increased risk of respiratory disorders, and this risk is likely to be greater than the risk associated with e-cigarettes.[18]
In December 2016, Philip Morris International presented a multi-million page application to the US FDA for IQOS to be authorized to be sold as a modified risk tobacco product.[95] In March 2017, Philip Morris International submitted a premarket tobacco product application regarding its IQOS product to the US FDA.[133] In December 2017, Reuters published documents and testimonies of former employees detailing irregularities in the clinical trials conducted by Philip Morris International for the approval of the IQOS product by the US FDA.[134] This investigative found that Philip Morris International was campaigning to obstruct or at a minimum diminish the provisions set forth under the WHO Framework Convention on Tobacco Control (FCTC).[119] The advisory panel appointed by the US FDA reviewed Philip Morris International's application in January 2018.[135] The advisory panel made recommendations about the application to the FDA in January 2018.[135] The FDA granted permission to Philip Morris International to sell IQOS in the US on April 30, 2019, which also requires the company to follow strict marketing restrictions.[136] IQOS formally launched in the US in October 2019.[137]
On July 7, 2020, the US FDA announced its decision, which authorized "exposure modification" orders, that permits Philip Morris International to market IQOS in the US using the claim, among other claims, that IQOS "significantly reduces the production of harmful and potentially harmful chemicals".[138] The US FDA dismissed the claims that IQOS is less dangerous than other tobacco products or lowers one's health risk.[note 4][15] The US FDA states that even with this decision, these products are not safe nor "FDA approved."[138] The WHO states that "Given that health may be affected by exposure to additional toxins [toxicants] when using HTPs, claims that HTPS reduce exposure to harmful chemicals relative to conventional cigarettes may be misleading."[15]
On September 29, 2021, the US International Trade Commission made a decision that Philip Morris International and its US partner Altria must desist from selling and importing the IQOS product in the US as a consequence of a patent case filed by R.J. Reynolds.[139] The US International Trade Commission determined that the IQOS product violated two patents owned by R.J. Reynolds.[139] Philip Morris International stated it intends to appeal the US International Trade Commission's ruling.[140]
iSmoke OneHitter
The iSmoke OneHitter by iSmoke can be used as a loose-leaf tobacco vaporizer or for use with waxy oils.[141] It is described as a "heat, not burn" tobacco vaporizer,[142] and was launched in 2015.[143] It has a chamber that can be filled with up to 800 milligrams of tobacco.[141]
IUOC 2
The IUOC 2 is marketed by Shenzhen Yukan Technology Co., Limited, of China.[144] The The heat-not-burn device can use any pack of 20 cigarettes on a single battery charge and does not use tobacco-filled cartridges.[144] The user inserts the entire cigarette into the device.[144] It is an updated version over the original IUOC and was formally launched in 2018 at InterTabac in Germany.[144]
lil

The lil is a heat-not-burn cigarette product that heats a cigarette stick with a circular blade[145] that was launched by Korea Tobacco & Ginseng Corporation on November 20, 2017.[146] A two-hour battery charge lasts for up to 20 cigarette sticks, its refills are cheaper than the IQOS and glo, and will fit in the IQOS product, though they do not recommend doing so for safety reasons.[145] Its lil Hybrid 2.0 automatically heats the tobacco stick following its insertion into the product.[147] In January 2020, Philip Morris International and Korea Tobacco & Ginseng Corporation enterd into a three-year partnership for Philip Morris International to distribute Korea Tobacco & Ginseng Corporation's products internationally.[147]
Mok
In May 2019, China Tobacco debuted the Mok heat-not-burn device in Korea.[148] According to the company, Mok is more compact and weighs less than other products such as glo, IQOS, and lil.[148] The sticks, which are known as Coo, are longer and wider than tobacco sticks from other companies.[148]
Pax vaporizers

In 2010 Ploom launched a butane-powered product used to heat tobacco or botanical products.[149] Later models replaced the butane heating with an electric system.[150] After its initial partnership with Japan Tobacco was abandoned, the company became known as Pax Labs.[151]
Ploom later rebranded to Pax Labs and began selling vaporizers.[152] The Pax 2 uses loose plant material such as tobacco or cannabis[153] and it surface remains cool, while the oven heats to temperatures up to 455 °F.[154] It has four temperature options.[154] Pax Era uses cannabis concentrates.[155] The Pax 3 takes 15 seconds to heat up[108] and can be used to heat cannabis flower.[156]
Ploom vaporizers
In March 2016, Japan Tobacco released its heat-not burn device Ploom Tech in Fukuoka, Japan.[157] Its Ploom Model One and Model Two has been withdrawn from the US.[104] The Ploom brand remained with Japan Tobacco and the product has been replaced with Ploom Tech, where an aerosol passes through a capsule of granulated tobacco leaves.[158] The Ploom brand uses aluminum capsules called Vapodes, where tobacco can heat up to 180 °C.[8] Because the Ploom Tech heats up more, it may generate more harmful emissions.[8] In January 2019, Japan Tobacco introduced Ploom TECH+ and Ploom S in Tokyo, Japan.[159]
Sales expanded throughout Japan in 2017.[160] Japan Tobacco intended to spend $500 million to increase their heated tobacco manufacturing capacity by late 2018.[161] Studies have not been conducted on Japan Tobacco International's Ploom product as of 2017[update].[67]
Pulze
In 2018, it was reported that Imperial Brands was developing a heated tobacco product named Pulze.[162] The Pulze has not been launched as of May 2017[update].[163]
TEEPS
In December 2017, Philip Morris International launched TEEPS in the Dominican Republic.[164] It is a heat-not-burn product that looks similar to a traditional cigarette.[164] Instead of an electrically controlled heating system, it uses a carbon heat source that, once lit, passes heat to a processed tobacco plug.[165]
Comparison to mainstream smoke of traditional cigarettes
Contents of selected analytes in the mainstream aerosol of a heated tobacco product compared to the mainstream smoke of traditional cigarettes.[12] The highest and lowest values in two different types of tobacco sticks and traditional cigarettes were given by Mallock et al. and Counts et al. respectively.[12] Column 5 shows the reduction of the analytes in the mainstream aerosol of the heated tobacco product compared with traditional cigarettes by percentage.[12]
| Tobacco sticks (Mallock et al. 2018; [15]) | Traditional Cigarettes (Counts et al. 2005; [18]) | Reduction | ||
|---|---|---|---|---|
| Parameter | Unit | Min.–Max. | Min.–Max. | % |
| Puff count | puff/stick | 12 | 5.5–13.6 | – |
| TPM | mg/stick | 51.2–52.6 | 27.5–60.9 | – |
| Nicotine | mg/stick | 1.1 | 1.07–2.70 | – |
| Water | mg/stick | 28.0–31.7 | 9.8–21.4 | – |
| NFDPM | mg/stick | 19.8–21.6 | 16.3–37.6 | – |
| Acetaldehyde | µg/stick | 179.4–183.5 | 930–1540 | 80.5–88.2 |
| Acrolein | µg/stick | 8.9–9.9 | 89.2–154.1 | 89.5–93.9 |
| Formaldehyde | µg/stick | 4.7–5.3 | 29.3–130.3 | 82.9–96.2 |
| Crotonaldehyde | µg/stick | <3.0 | 32.7–70.8 | – |
| 1.3-Butadiene | µg/stick | 0.20–0.2 | 77.0–116.7 | 99.7–99.8 |
| Benzine | µg/stick | 0.5–0.6 | 49.7–98.3 | 98.8–99.4 |
| Isoprene | µg/stick | 1.8–2.1 | 509–1160 | 99.6–99.8 |
| Styrene | µg/stick | 0.5 | 15.4–33.3 | 96.9–98.6 |
| Toluene | µg/stick | 2.0–2.2 | 86.2–176.2 | 97.6–98.8 |
Tobacco stick, i. e. for heated tobacco products: a tobacco stick; for traditional cigarette: a cigarette.[12]
All values were generated using the Health Canada Intense (HCI) puffing conditions.[12]
TPM = total particulate matter, and NFDPM = nicotine-free dried particulate matter.[12]
Prevalence
As of 2017[update], heated tobacco products are being introduced in markets around the world,[166] and since 2020, they have been sold in more than 50 countries.[167] They are not as globally popular as the e-cigarette.[25] As of 2018[update], the IQOS is the most popular product,[25] and was authorized for marketing by the US FDA in the US on April 30, 2019.[136] In 2021, the IQOS product is no longer permitted to be sold and imported into the US.[139]
As of April 2018[update], the industry has been rapidly introducing new heated tobacco products.[29] Heated tobacco products were first sold in Japan,[168] and several brands have been marketed there since 2014.[166] Because Japan has tight regulations on nicotine-containing liquids, this has hampered the sales of e-cigarettes there, which makes it an ideal location for testing the latest heated tobacco technology.[169]
The share of the market in South Korea for heated tobacco products has surged at least five-fold during the last two years leading up to 2019.[170] As of early 2018, these products are not sold in France.[8]
Tobacco industry leaders had predicted that heated tobacco products may displace traditional cigarette smoking and, by extension, tobacco control strategies typically framed around cigarettes.[166] As of 2017[update], the worldwide market for heated tobacco products is $5 billion, while it is $680 billion for traditional tobacco products.[171] In 2018, British American Tobacco states that the surge in sales of heated tobaccos products in Japan has stalled, casting uncertainty that these types of products can counter the drop in traditional cigarette use.[172] Coverage of the hospitalizations and deaths linked to vaping-induced lung injuries in 2020 could result in people to experiment with unconventional forms of nicotine products, such as heated tobacco products.[48]
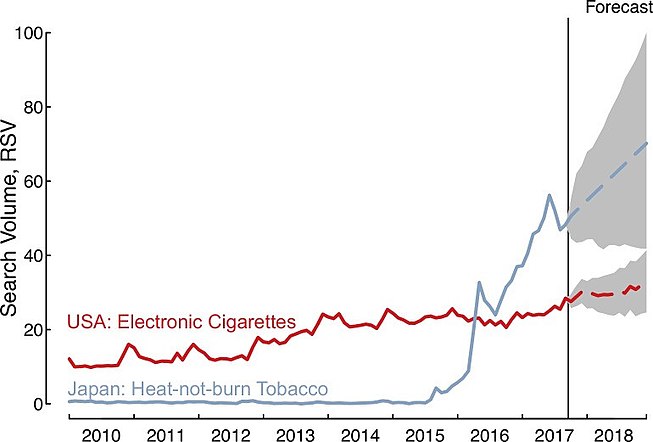
Since the introduction of Philip Morris International's IQOS brand in select Japanese cities in November 2014, web searches in Japan for "heat-not-burn products" (sometimes used as a marketing term for heated tobacco products[6]) increased substantially; average monthly searches rose 1,426% (95% CI: 746–3,574) during 2015–2016, and they continued to grow an additional 100% (95% CI: 60–173) between 2016 and 2017; in practical terms, there are now between 5.9 and 7.5 million heat-not-burn related Google searches in Japan each month based on the latest search estimates for September 2017.[166] Moreover, forecasts relying on the historical trend suggest heat-not-burn searches would increase an additional 32% (95%CI: -4 to 79) during 2018, compared with current estimates for 2017 (January–September).[166]
Queries for heat-not-burn in Japan occur more frequently than queries for e-cigarettes in the United States, with the Japanese heat-not-burn queries first eclipsing e-cigarette queries in April 2016.[166] Further, the change in average monthly queries for heat-not-burn in Japan between 2015 and 2017 was 399 (95% CI: 184–1,490) times larger than the change in average monthly queries for e-cigarettes in the United States over the same time period, increasing by 2,956% (95% CI: 1,729–7,304) compared with only 7% (95% CI: 3–13), which indicate that interest in heat-not-burn may outpace interest in e-cigarettes in the future.[166]
-
Google searches for heat-not-burn tobacco outpace past rise of electronic cigarettes.[166] The above figure shows the Relative Search Volume (scaled from 0–100 and adjusted for the number of total Google search volumes per month in Japan and the USA) for heat-not-burn and e-cigarette products.[166]
Heated tobacco product demand presents a host of tobacco control challenges similar to e-cigarettes and new challenges specific to these products.[166] They have been advertised as reduced-risk tobacco products in their Japanese test market.[166]
Marketing

The term "heat-not-burn" refers to tobacco heated (at ~350 °C) by an electrically powered element or carbon instead of being combusted (at ~800 °C).[25] Terms used in marketing for cigarette-like products that "heat rather than burn" refer to them as "reduced risk" and "innovative".[107] The term "heat-not-burn" has been used as a marketing slogan.[127] The tobacco industry has described them as "not-burned" (heat-not-burn), though it has backtracked from this claim as of 2018[update].[8] Heated tobacco products are not typically marketed as a harmless substitute to smoking,[63] though they have been marketed as a "smoke-free" alternative to traditional cigarettes, and promoted as a way to lower risk from smoking.[173] The IQOS product has been advertised as emitting "no smoke".[174] It is expected that the promotion associated with these products will worsen the worldwide tobacco risk.[8]
Companies employ similar strategies previously used for traditional cigarettes, such as marketing through a variety of outlets, including celebrity endorsements.[175] A 2020 World Health Organization reports states that "Marketing channels include the internet, promotional events, flagship stores, supermarkets, shopping malls, social media etc., especially with an eye towards markets in low- and middle-income countries."[45] "The tobacco industry has opened heated tobacco product flagship stores, cafes and sponsored public events such as concerts and car races around the world, which is alarming," said Judith Mackay, director of the Asian Consultancy on Tobacco Control.[175] In Japan, IQOS has been marketed as a clean, chic, and pure product, which resonated well in Japan given the strong cultural values of order, cleanliness, quality, and respect for others.[176] Japanese IQOS users are reportedly motivated to use IQOS to socialize with non-smokers.[176]
Internal documents and statements by Philip Morris International researchers have contradicted Philip Morris International's claims about reduced harm in regard to the IQOS product.[95] For example, in 2018, four Philip Morris International researchers who had worked for the company stated that the lowered levels of certain substances produced by the IQOS did not automatically translate into the product being safer, even though Philip Morris International stated that the IQOS is safer than traditional cigarettes, as 58 substances in IQOS aerosols were found at lower levels than in cigarette smoke.[95]
The tobacco industry claims that traditional cigarette smokers will switch to heated tobacco products; however, current IQOS users are more likely to smoke and/or use e-cigarettes as well.[176] Among those who have tried or intend to try IQOS, never-smokers equal or outnumber smokers.[176] A review of Philip Morris International's own research found that smokers did not understand "switching completely" and that IQOS users are not likely to switch completely.[176]
Philip Morris International has used various approaches to promote IQOS.[176] The evidence indicates that Philip Morris International is promoting IQOS in places where it is subject to different regulations than combustible tobacco.[177] Since 2017, Philip Morris International has been promoting its IQOS product in Europe and Asia,[132] where IQOS products are sold as an alternative to regular cigarettes.[178] Since April 2017, Philip Morris International has been promoting its IQOS in Ontario, Canada.[177] A 2018 study in Canada found that IQOS boutique stores use aggressive promotional activities, including deals involving exchanging cigarette packs or lighters for an IQOS device, social events, and membership programs, with signage reading "Building a Smoke-Free Future" and sales representatives regularly smoking IQOS.[176] On August 1, 2019, researchers found a paid advertisement for IQOS showing up in Google search results for the term "heat-not-burn."[179] The advertisement incorrectly insinuated that the FDA endorsed its product and portrayed IQOS as a 'smoking alternative', which may insinuate a reduced harm, which is a breach of US FDA regulations.[179] Viewers were asked to accept a message from a subsidiary of Philip Morris International stating that the website was not for advertising or marketing, despite the website being used to promote IQOS.[179]
There has been significant controversy surrounding the marketing and use of these products.[180] The tobacco companies are using a series of claims in the marketing of heated tobacco products.[29] In both websites and statements to the media and investors, heated tobacco products are presented as less harmful but not risk-free.[29] In a few instances, marketing materials claim that heated tobacco products are potentially helpful to smokers who want to quit.[29] Some media accounts that announced product launches state that heated tobacco products reduce the levels of harmful tobacco components by 90–95% compared to traditional cigarettes, while others emphasize the lack of odor or visible emissions as part of marketing campaigns; as of April 2018[update], there is no evidence to confirm the former claim.[29] Other marketing claims highlight that these products produce no smoke (i.e., "smoke-free").[29] In 2016, the introduction of the latest generation of heated tobacco products appeared to be the latest industry attempt in the decades-old tobacco industry strategy of working to create partnerships with governments and health advocates, presenting these alleged "harm reduction" products as an option to address the tobacco epidemic.[29]
| Product name | Marketing terms | Product appeal |
|---|---|---|
| IQOS | Reduced risk product, innovative | Clean (white, bright blue), stylish, elegant |
| Revo | Reduced risk | Similar sized package as traditional cigarette, white or light grey or gold |
| PAX 2 | Smaller, smarter, sleeker | Design, elegant and fun |
| iFuse | Reduced risk | Packed as traditional cigarette, stylish |
The tobacco industry's use of the "harm reduction" framework also serves to fracture the tobacco control movement, leaving it without a unified voice to communicate with the public, the media and with policymakers on the strategies to advance tobacco control.[29] The concept of harm reduction has traditionally been embraced in several public health fields such as clean needles for injectable drug use and has been explored by some tobacco control experts in the past, with enthusiasm for the possibility of harm reduction growing with the widespread availability of e-cigarettes in certain markets.[29] The tobacco industry frames harm reduction as a common ground with health advocates and a possible entry point to influence legislation and regulation of tobacco products.[29]
The tobacco companies use heated tobacco products as part of their broader political and public relations activities to position them as 'partners' to address the tobacco epidemic rather than as the vectors that are causing it.[29] This is a similar strategy previously used by the tobacco industry to promote itself as a partner of public health in reducing the harms of tobacco, while obfuscating the scientific evidence pointing that harm reduction is achieved through tobacco control policies that decrease consumption.[29] The tobacco industry promotes heated tobacco products as a safer alternative to traditional cigarettes with misleading marketing sustained by studies with a conflict of interest.[30] As of September 2017[update], 19 out of 27 of the PubMed-indexed citations on "heat-not-burn" are produced by Philip Morris International or other tobacco businesses.[20] As of October 2019[update], 52% of the research on heated tobacco products have been sponsored by tobacco businesses.[40]
Philip Morris International has been cognizant of targeting policymakers, press, and merchants.[176] For example, Philip Morris International's "Smoke-Free Israel" campaign targeted policy makers and the general public, emphasizing its alleged potential for harm reduction and the justification for regulation and policies different from traditional cigarettes (e.g., taxation, smoking and advertising bans, health warning labels), similar to its situation in Italy.[176] The campaign included meetings with government officials and policymakers, as well as a public campaign targeted young people, which emphasized IQOS's cleanliness Philip Morris International's campaign initially resulted in IQOS being exempt from tobacco regulations, which was later reversed, after fightback from a strong public health coalition.[176] Other data indicate that Philip Morris International aligns with press to promote their campaign messages and with merchants by providing demonstrations and free samples.[176]
In May 2019, Reuters reported that Philip Morris International was using young online personalities as ambassadors for the brand and to promote IQOS to a young demographic.[181] In response, Philip Morris International halted its social media influencers marketing campaign globally.[181] One of the social media campaigns had been a paid post promoting IQOS by a 21-year-old woman in Moscow, "alongside often seductive photos of herself drinking wine, swimming and posing with little clothing in luxurious settings."[181] The company stated to Reuters that "Whilst the influencer in question is a legal age adult smoker, she is under 25 and our guidance called for influencers to be 25+ years of age. This was a clear breach of that guidance."[181] Matthew Myers, president of the Campaign for Tobacco-Free Kids, states that Philip Morris International "is changing their behavior only when caught red-handed."[181] Later on, in February 2020, Philip Morris International was partnering with social media influencers in Ukraine to promote its IQOS brand.[75] Subsequent to critics contending that IQOS focuses on Ukraine's youth, the chief manager of Philip Morris International in Ukraine, Michalis Alexandrakis, stated: "We know very well that the problem with the youth is not related to IQOS, it is not related to 'heat-not-burn' products, it is related to vaping. It has nothing to do with IQOS. That is what we all know but we keep repeating IQOS."[182]
In regard to Philip Morris International's IQOS product marketing campaign, a 2020 Stanford report found that "Its use of youth-oriented social media channels, trendy pop music festivals and celebrity influencers are mis-aligned with their commitment to exclusive 'adult smoker' targeting."[183] A 2020 Tobacco Control report states that "Philip Morris has strongly lobbied the Australian government to legalise heated tobacco products, while simultaneously making plans to sell IQOS at young adult-friendly premises such as bars, clubs and pubs if its proposed legislative changes are made."[184]
An entire page advertisement appeared in The Wall Street Journal on June 29, 2021 that was paid for by Philip Morris International in which they discussed "misinformation and disinformation" in regard to those who disapprove of heated tobacco products.[185]
Regulation
Heated tobacco products are subject to different regulations than traditional cigarettes in many countries.[186] A handful of countries have banned heated tobacco products and in other places they are regarded as tobacco products, unconventional tobacco products, smokeless tobacco products, or e-cigarettes.[187] Many countries do not have regulations explicately covering heated tobacco products in their tobacco control laws.[188] A 2018 review states, the IQOS product emits smoke, and therefore, must adhere to the regulations governing newly introduced smoked tobacco products.[8]
Companies have attempted to circumvent the laws that are designed to regulate tobacco products, which includes heated tobacco products, by selling each component of each product individually.[186] Current smoking bans may not have been extended to include them[31] and in the majority of the countries in which they have been sold, they have been taxed at a lower rate than traditional cigarettes.[189] Tobacco companies have used these products to seek exemptions and relaxations of existing tobacco control policies,[190] and have used them in attempts to influence regulatory policy to sustain and increase their clientele in the midst of decreasing cigarette usage.[190] As of 2019[update], 49 countries have permitted the sale of IQOS.[137]
In the United States, these products fall under the jurisdiction of the US Food and Drug Administration as amended by the Family Smoking Prevention and Tobacco Control Act of 2016[79] and are classified as non-combusted cigarettes there.[5] In 2016, Action on Smoking and Health stated that "unless and until independent evidence shows that IQOS and similar products are substantially less harmful than smoking then these products should be regulated in the same way as other tobacco products."[39] In 2020, the World Health Organization recommended that heated tobacco products ought to be regulated just the same as traditional tobacco products.[187] In 2017, Mitchell H. Katz, director of the Los Angeles County Health Agency, wrote: "There is concern that heat-not-burn tobacco will skirt local ordinances that prevent smoking in public areas."[31] Tobacco control activist Stanton Glantz stated that the US FDA should halt new tobacco products until tobacco companies stop selling traditional cigarettes.[191] In 2017, Auer et al. and colleagues recommended that indoor-smoking bans for traditional cigarettes be extended to include heated tobacco products.[127] In 2018, Bialous et al. and Glantz et al. recommended that the marketing of these products, and claims being made about them, need to be regulated.[29]
Advertisement for the IQOS product itself is not regulated under the European Union Tobacco Products Directive, though the directive may apply to advertising for the IQOS' tobacco stick.[104] The UK government has been looking into creating a separate category for taxing heated tobacco products.[192]
In Canada, IQOS is regulated as a tobacco product.[177]
In November 2020, the Supreme Court of Justice of the Nation allowed the marketing of heated tobacco products in Mexico.[30]
Due to the alleged belief in heated tobacco harm reduction in Italy, heated tobacco products are exempted from the fiscal regimes of tobacco products.[61] Taxes on them are reduced as much as e-cigarettes, or half of traditional cigarettes.[61] Moreover, the enforcement of various tobacco control regulations is only minimally adopted for heated tobacco products in Italy: health warnings are required to cover only 30% of the heated tobacco product packaging (instead of 65% for traditional cigarettes), without pictorial images; comprehensive smoke-free regulations prohibiting smoking in all public places and workplaces do not apply to heated tobacco products; and advertising and promotions are not banned for them.[61] Epidemiologist Xiaoqiu Liu et al. noted the lax enforcement over heated tobacco products have been exploited by the presence in several strategic Italian cities of the "IQOS embassy" and "IQOS boutiques"—fancy concept stores where IQOS is promoted as a status symbol and free samples are given—and believe the most recognized tobacco control policies in Italy (i.e., price/tax increase, smoking bans, advertising bans, and health warnings) have been compromised by heated tobacco products.[61]
Heated tobacco products are not restricted for sale in Israel by the Ministry of Health.[193] The Justice Ministry in Israel agreed with the view of three voluntary organizations that the IQOS is a tobacco product, and that it should be regulated in the same manner as tobacco products.[194] In Israel the IQOS is taxed at the same rate as traditional cigarettes.[195]
The nicotine-containing e-liquid used in e-cigarettes is considered a pharmaceutical ingredient in Japan, where it is restrictly regulated,[196] while Ploom, IQOS, and glo fall under the Tobacco Business Act as tobacco products in Japan because they consist of tobacco leaf.[26] Ploom and IQOS are governed by the Tobacco Industries Act regulations as tobacco products in Japan.[70] In 2017, the Liberal Democratic Party in Japan had planned to deliberate increasing the tax rate on heated tobacco products the following year in April 2018.[197]
Electronic tobacco products using dry material are regulated as e-cigarettes in South Korea by the Ministry of Health and Welfare,[198] which are regulated differently than traditional cigarettes for tax reasons.[199] As a result, IQOS are taxed at a lower rate when compared to the 75% incurred on normal cigarettes.[199] Emerging tobacco products are banned in Singapore by the Ministry of Health.[200] China plans to pass legislation to ban the sale of these products to minors, as of 2019.[201] Starting April 30, 2022 in Hong Kong, alternative smoking products are regulated within the Part 2 of Schedule 7 to the Smoking (Public Health) Amendment Ordinance 2021.[202] Import, promotion, production, sale or ownership for business use of alternative smoking products is not permissible and is actionable with a penalty of HK$50,000 and jail time of 6 months.[202]
After IQOS launched a marketing campaign in New Zealand in December 2016, the country's Ministry of Health stated in 2017 that the refill sticks are not legal for sale in New Zealand under the Smoke-free Environments Act 1990.[203] A representative for the company in New Zealand stated that IQOS products comply with the Smoke-Free Environments Act.[204] Three meetings between Ministry of Health officials and people from the tobacco industry were held from May 30, 2017 through June 2, 2017 to "discuss regulation of new tobacco and nicotine-delivery products".[205] In August 2017, the government stated they would initiate a review process before products are sold for heated tobacco products such as IQOS.[205] In 2018, Philip Morris International and the Ministry of Health were in a legal dispute over the legality of selling IQOS in New Zealand.[206] In March 2018, the New Zealand court decided that the HEETs sticks used in the IQOS product are legal to sell in the country.[207] Individuals can import heated tobacco products to New Zealand for personal use.[208]
Environmental impact
Heated tobacco products threaten the environment.[209] A 2020 Public Health Law Center report stated "New products such as e-cigarettes, or heated cigarette products like Iqos, will increase the overall supply of e-waste. It is most likely impossible to create any e-cigarette without a battery, poisonous liquid, metals and plastics joined in small devices, each of which cannot be recycled or disposed of responsibly."[32]
Other names
A heated tobacco product (HTB),[40] is also variously known as heat-not-burn product,[210] heat-not-burn device,[211] heat-not-burn tobacco product (HNB),[20] Heat-not-Burn tobacco product,[212] "heat-not-burn" product,[178] heat-not-burn (HNB) tobacco cigarette,[127] heat-not-burn cigarette (HC),[213] heat-not-burn cig,[214] HNB tobacco product,[20] HnB tobacco product,[38] HNB product,[215] HnB product,[38] heat-not-burn tobacco device,[216] heat-not-burn system,[43] HnB system,[38] heat-not-burn (HnB) product,[163] heat-not-burn (HNB) device,[215] heat-not-burn device,[43] HNB device,[215] HnB tobacco device,[12] HnB device,[111] heat-not-burn vapor device,[157] heat-not-burn vaporizer,[196] HNB cigarette,[217] heated cigarette,[218] HTP cigarette,[219] cigarette-like product,[107] mini-cigarette,[8] next-generation product (NGP),[220] emerging tobacco related products (ETRPs),[221] modified-risk tobacco product,[222] reduced-harm tobacco product,[222] tobacco-heating product,[172] heated-tobacco product,[223] electronic heated tobacco product,[77] heated smoking device,[224] electronically-heated cigarette smoking system (EHCSS),[33] electrically heated cigarette smoking system,[95] electrically heated tobacco system,[225] non-combusted cigarette,[5] non-combusted tobacco product,[5] tobacco heating cigarette,[34] tobacco heating product[226] (THP),[227] tobacco heating system[228] (THS),[44] smokeless cigarette,[83] smokeless tobacco stick,[229] tobacco stick product,[173] loose-leaf tobacco vaporizer (LLTV),[79] tobacco vaporizer,[230] or T-vapor.[231]
Notes
- ↑ There are a variety of products colloquially called heated tobacco products and heat-not-burn products that do not appear to fit easily into universally agreed upon product categories.[5] Products currently sold in global markets may function in various ways.[5] For example, these products heat to various temperatures, can contain dry, moist, or liquid tobacco ingredients, and appear in a wide variety of shapes.[5] Heated tobacco products heat actual tobacco leaf.[6] By contrast, e-cigarettes heat liquids that typically contain nicotine derived from tobacco, as well as flavorings and other ingredients.[6]
- ↑ The World Health Organization states that "HNB tobacco products are not e-cigarettes. HNB tobacco products heat tobacco to generate nicotine. E-cigarettes heat e-liquid, which may or may not contain nicotine and in most cases do not contain tobacco."[7]
- ↑ A "throat-hit" is a sensorial experience that is a form of airway irritation apparently resulting from nicotine use.[102]
- ↑ The US FDA states that "There are two types of MRTP orders the FDA may issue: a 'risk modification' order or an 'exposure modification' order.[138] The company had requested both types of orders for the IQOS Tobacco Heating System.[138] After reviewing the available scientific evidence, public comments and recommendations from the Tobacco Products Scientific Advisory Committee, the FDA determined that the evidence did not support issuing risk modification orders at this time but that it did support issuing exposure modification orders for these products."[138]
Bibliography
- "Heated tobacco products: a brief (2020)". World Health Organization. 2020. pp. 1–11.
- "Heated tobacco products: information sheet - 2nd edition". World Health Organization. 10 July 2020. pp. 1–4.
- "WHO Report on the Global Tobacco Epidemic, 2019" (PDF). World Health Organization. July 2019. pp. 1–209.
- McNeill, A; Brose, LS; Calder, R; Bauld, L; Robson, D (February 2018). "Evidence review of e-cigarettes and heated tobacco products 2018" (PDF). UK: Public Health England. pp. 1–243.
- "Regulatory Impact Statement: Regulation of smokeless tobacco and nicotine-delivery products" (PDF). Ministry of Health (New Zealand). 2017. pp. 1–52. Archived from the original (PDF) on 17 June 2019.
- "Further development of the partial guidelines for implementation of Articles 9 and 10 of the WHO FCTC" (PDF). World Health Organization. 12 July 2016. pp. 1–11.
- "Alternatieve tabaksproducten: harm reduction?" [Alternative tobacco products: harm reduction?] (PDF) (in Dutch). Netherlands National Institute for Public Health and the Environment. 2016. pp. 1–66.
{{cite web}}: CS1 maint: unrecognized language (link)
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Mallock, Nadja; Pieper, Elke; Hutzler, Christoph; Henkler-Stephani, Frank; Luch, Andreas (10 October 2019). "Heated Tobacco Products: A Review of Current Knowledge and Initial Assessments". Frontiers in Public Health. 7. doi:10.3389/fpubh.2019.00287. PMC 6795920. PMID 31649912.
 This article incorporates text by Nadja Mallock, Elke Pieper, Christoph Hutzler, Frank Henkler-Stephani, and Andreas Luch available under the CC BY 4.0 license.
This article incorporates text by Nadja Mallock, Elke Pieper, Christoph Hutzler, Frank Henkler-Stephani, and Andreas Luch available under the CC BY 4.0 license.
- ↑ 2.0 2.1 Upadhyay, Swapna; Rahman, Mizanur; Johanson, Gunnar; Palmberg, Lena; Ganguly, Koustav (2 August 2023). "Heated Tobacco Products: Insights into Composition and Toxicity". Toxics. 11 (8): 667. doi:10.3390/toxics11080667. PMC 10459283. PMID 37624172.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Swapna Upadhyay, Mizanur Rahman, Gunnar Johanson, Lena Palmberg, Koustav Ganguly available under the CC BY 4.0 license.
This article incorporates text by Swapna Upadhyay, Mizanur Rahman, Gunnar Johanson, Lena Palmberg, Koustav Ganguly available under the CC BY 4.0 license.
- ↑ 3.0 3.1 Caleb Mutua, David; Gretler, Corinne (27 August 2021). "Philip Morris to Court ESG Investors Whose Mantra Is Shunning It". Bloomberg News.
- ↑ 4.0 4.1 4.2 4.3 WHO-Brief 2020, p. 6.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "How are Non-Combusted Cigarettes, Sometimes Called Heat-Not-Burn Products, Different from E-Cigarettes and Cigarettes?". United States Food and Drug Administration. 1 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 "Heated Tobacco Products". Centers for Disease Control and Prevention. 16 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 "Heat-Not-Burn tobacco products information sheet". World Health Organization. May 2018.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 8.14 8.15 8.16 8.17 8.18 8.19 8.20 8.21 8.22 8.23 8.24 8.25 8.26 8.27 8.28 8.29 8.30 8.31 8.32 8.33 8.34 8.35 8.36 8.37 Dautzenberg, B.; Dautzenberg, M.-D. (2018). "Le tabac chauffé : revue systématique de la littérature" [Systematic analysis of the scientific literature on heated tobacco]. Revue des Maladies Respiratoires (in French). 36 (1): 82–103. doi:10.1016/j.rmr.2018.10.010. ISSN 0761-8425. PMID 30429092.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Uguna, Clement N.; Snape, Colin E. (5 July 2022). "Should IQOS Emissions Be Considered as Smoke and Harmful to Health? A Review of the Chemical Evidence". ACS Omega. 7 (26): 22111–22124. doi:10.1021/acsomega.2c01527. PMC 9260752. PMID 35811880.
 This article incorporates text by Clement N. Uguna and Colin E. Snape available under the CC BY 4.0 license.
This article incorporates text by Clement N. Uguna and Colin E. Snape available under the CC BY 4.0 license.
- ↑ Başaran, Rahman; Güven, Naile Merve; Eke, Benay Can (11 July 2019). "An Overview of iQOS® as a New Heat-Not-Burn Tobacco Product and Its Potential Effects on Human Health and the Environment". Turkish Journal of Pharmaceutical Sciences. 16 (3): 371–374. doi:10.4274/tjps.galenos.2018.79095. PMID 32454738.
- ↑ 11.0 11.1 11.2 11.3 11.4 Caputi, TL (24 August 2016). "Industry watch: heat-not-burn tobacco products are about to reach their boiling point". Tobacco Control. 26 (5): 609–610. doi:10.1136/tobaccocontrol-2016-053264. PMID 27558827. S2CID 46170776.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 12.15 12.16 12.17 12.18 12.19 12.20 12.21 12.22 12.23 12.24 12.25 12.26 12.27 12.28 12.29 12.30 12.31 12.32 12.33 Pieper, Elke; Mallock, Nadja; Henkler-Stephani, Frank; Luch, Andreas (2018). "Tabakerhitzer als neues Produkt der Tabakindustrie: Gesundheitliche Risiken" ["Heat not burn" tobacco devices as new tobacco industry products: health risks]. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (in Deutsch). 61 (11): 1422–1428. doi:10.1007/s00103-018-2823-y. ISSN 1436-9990. PMID 30284624. S2CID 52915909.
 This article incorporates text by Elke Pieper, Nadja Mallock, Frank Henkler-Stephani, and Andreas Luch available under the CC BY 4.0 license.
This article incorporates text by Elke Pieper, Nadja Mallock, Frank Henkler-Stephani, and Andreas Luch available under the CC BY 4.0 license.
- ↑ 13.0 13.1 13.2 MHNZ 2017, p. 4.
- ↑ 14.0 14.1 "Philip Morris International Launches New IQOS Iluma in Japan". Tobacco Business Magazine. 17 August 2021.
- ↑ 15.0 15.1 15.2 15.3 "WHO statement on heated tobacco products and the US FDA decision regarding IQOS". World Health Organization. 27 July 2020.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 WHO 2020, p. 1.
- ↑ 17.0 17.1 17.2 WHO 2016, p. 6.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 18.7 18.8 18.9 Znyk, Małgorzata; Jurewicz, Joanna; Kaleta, Dorota (21 June 2021). "Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review". International Journal of Environmental Research and Public Health. 18 (12): 6651. doi:10.3390/ijerph18126651.
 This article incorporates text by Małgorzata Znyk, Joanna Jurewicz, and Dorota Kaleta available under the CC BY 4.0 license.
This article incorporates text by Małgorzata Znyk, Joanna Jurewicz, and Dorota Kaleta available under the CC BY 4.0 license.
- ↑ 19.0 19.1 19.2 McNeill 2018, p. 220.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 20.8 20.9 Jenssen, Brian P.; Walley, Susan C.; McGrath-Morrow, Sharon A. (2017). "Heat-not-Burn Tobacco Products: Tobacco Industry Claims No Substitute for Science". Pediatrics. 141 (1): e20172383. doi:10.1542/peds.2017-2383. ISSN 0031-4005. PMID 29233936. S2CID 41704475.
- ↑ 21.0 21.1 21.2 21.3 21.4 McNeill 2018, p. 23.
- ↑ 22.0 22.1 WHO 2019, p. 54.
- ↑ 23.0 23.1 Pisinger, Charlotta (2019). "ERS Position Paper on Heated Tobacco Products". European Respiratory Society.
- ↑ 24.0 24.1 24.2 Chaturvedi, Pankaj; Mishra, Aseem; Datta, Sourav; Sinukumar, Snita; Joshi, Poonam; Garg, Apurva (2015). "Harmful effects of nicotine". Indian Journal of Medical and Paediatric Oncology. 36 (1): 24. doi:10.4103/0971-5851.151771. ISSN 0971-5851. PMC 4363846. PMID 25810571.
- ↑ 25.00 25.01 25.02 25.03 25.04 25.05 25.06 25.07 25.08 25.09 25.10 25.11 25.12 25.13 25.14 25.15 25.16 25.17 25.18 Li, Gerard; Saad, Sonia; Oliver, Brian; Chen, Hui (2018). "Heat or Burn? Impacts of Intrauterine Tobacco Smoke and E-Cigarette Vapor Exposure on the Offspring's Health Outcome". Toxics. 6 (3): 43. doi:10.3390/toxics6030043. ISSN 2305-6304. PMC 6160993. PMID 30071638.
 This article incorporates text by Gerard Li, Sonia Saad, Brian G. Oliver, and Hui Chen available under the CC BY 4.0 license.
This article incorporates text by Gerard Li, Sonia Saad, Brian G. Oliver, and Hui Chen available under the CC BY 4.0 license.
- ↑ 26.0 26.1 26.2 Tabuchi, Takahiro; Gallus, Silvano; Shinozaki, Tomohiro; Nakaya, Tomoki; Kunugita, Naoki; Colwell, Brian (2018). "Heat-not-burn tobacco product use in Japan: its prevalence, predictors and perceived symptoms from exposure to secondhand heat-not-burn tobacco aerosol". Tobacco Control. 27 (e1): e25–e33. doi:10.1136/tobaccocontrol-2017-053947. ISSN 0964-4563. PMC 6073918. PMID 29248896.
- ↑ 27.0 27.1 27.2 27.3 Marynak, Kristy L.; Wang, Teresa W.; King, Brian A.; Agaku, Israel T.; Reimels, Elizabeth A.; Graffunder, Corinne M. (2018). "Awareness and Ever Use of "Heat-Not-Burn" Tobacco Products Among U.S. Adults, 2017". American Journal of Preventive Medicine. 55 (4): 551–554. doi:10.1016/j.amepre.2018.04.031. ISSN 0749-3797. PMID 30033025.
- ↑ 28.00 28.01 28.02 28.03 28.04 28.05 28.06 28.07 28.08 28.09 28.10 "Toxicological evaluation of novel heat-not-burn tobacco products – non-technical summary" (PDF). Committee on Toxicity. 11 December 2017. pp. 1–4.
- ↑ 29.00 29.01 29.02 29.03 29.04 29.05 29.06 29.07 29.08 29.09 29.10 29.11 29.12 29.13 29.14 29.15 29.16 29.17 29.18 29.19 29.20 Bialous, Stella A; Glantz, Stanton A (2018). "Heated tobacco products: another tobacco industry global strategy to slow progress in tobacco control". Tobacco Control. 27 (Suppl 1): s111–s117. doi:10.1136/tobaccocontrol-2018-054340. ISSN 0964-4563. PMC 6202178. PMID 30209207.
 This article incorporates text by Stella A Bialous and Stanton A Glantz available under the CC BY 4.0 license.
This article incorporates text by Stella A Bialous and Stanton A Glantz available under the CC BY 4.0 license.
- ↑ 30.0 30.1 30.2 30.3 30.4 30.5 Bravo-Gutiérrez, Omar Andrés; Falfán-Valencia, Ramcés; Ramírez-Venegas, Alejandra; Sansores, Raúl H.; Ponciano-Rodríguez, Guadalupe; Pérez-Rubio, Gloria (2021). "Lung Damage Caused by Heated Tobacco Products and Electronic Nicotine Delivery Systems: A Systematic Review". International Journal of Environmental Research and Public Health. 18 (8): 4079. doi:10.3390/ijerph18084079. ISSN 1660-4601. PMC 8070637. PMID 33924379.
 This article incorporates text by Omar Andrés Bravo-Gutiérrez, Ramcés Falfán-Valencia, Alejandra Ramírez-Venegas, Raúl H. Sansores, Guadalupe Ponciano-Rodríguez, and Gloria Pérez-Rubio1 available under the CC BY 4.0 license.
This article incorporates text by Omar Andrés Bravo-Gutiérrez, Ramcés Falfán-Valencia, Alejandra Ramírez-Venegas, Raúl H. Sansores, Guadalupe Ponciano-Rodríguez, and Gloria Pérez-Rubio1 available under the CC BY 4.0 license.
- ↑ 31.0 31.1 31.2 Rapaport, Lisa (26 May 2017). "'Heat-not-burn' cigarettes still release cancer-causing chemicals". Reuters.
- ↑ 32.0 32.1 "To End the Tobacco Industry's Pollution, Put an End to the Tobacco Industry". Public Health Law Center. 30 June 2020.
- ↑ 33.0 33.1 Lindson-Hawley, Nicola; Hartmann-Boyce, Jamie; Fanshawe, Thomas R; Begh, Rachna; Farley, Amanda; Lancaster, Tim (2016). "Interventions to reduce harm from continued tobacco use". Cochrane Database of Systematic Reviews. 10 (12): CD005231. doi:10.1002/14651858.CD005231.pub3. ISSN 1465-1858. PMC 6463938. PMID 27734465.
- ↑ 34.0 34.1 34.2 RIVM 2016, p. 36.
- ↑ 35.0 35.1 35.2 RIVM 2016, p. 5.
- ↑ 36.0 36.1 36.2 Górski, Paweł (2019). "E-cigarettes or heat-not-burn tobacco products — advantages or disadvantages for the lungs of smokers". Advances in Respiratory Medicine. 87 (2): 123–134. doi:10.5603/ARM.2019.0020. ISSN 2543-6031. PMID 31038725.
- ↑ 37.0 37.1 McNeill 2018, p. 219.
- ↑ 39.0 39.1 39.2 "ASH reaction to new Philip Morris IQOS 'heat not burn' product". ASH UK. 30 November 2016.
- ↑ 40.0 40.1 40.2 40.3 Jankowski, Mateusz; Brożek, Grzegorz; Lawson, Joshua; Skoczyński, Szymon; Majek, Paulina; Zejda, Jan (2019). "New ideas, old problems? Heated tobacco products – a systematic review". International Journal of Occupational Medicine and Environmental Health. 32 (5): 595–634. doi:10.13075/ijomeh.1896.01433. ISSN 1232-1087. PMID 31584041.
- ↑ Katz, Mitchell H. (July 2017). "No Smoke—Just Cancer-Causing Chemicals". JAMA Internal Medicine. 177 (7): 1052. doi:10.1001/jamainternmed.2017.1425. ISSN 2168-6106. PMID 28531245.
- ↑ Szumilas, Paweł; Wilk, Aleksandra; Szumilas, Kamila; Karakiewicz, Beata (6 February 2022). "The Effects of E-Cigarette Aerosol on Oral Cavity Cells and Tissues: A Narrative Review". Toxics. MDPI AG. 10 (2): 74. doi:10.3390/toxics10020074. ISSN 2305-6304. PMC 8878056. PMID 35202260.
 This article incorporates text by Paweł Szumilas, Aleksandra Wilk, Kamila Szumilas, and Beata Karakiewicz available under the CC BY 4.0 license.
This article incorporates text by Paweł Szumilas, Aleksandra Wilk, Kamila Szumilas, and Beata Karakiewicz available under the CC BY 4.0 license.
- ↑ 43.0 43.1 43.2 43.3 Kaur, Gurjot; Muthumalage, Thivanka; Rahman, Irfan (2018). "Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products". Toxicology Letters. 288: 143–155. doi:10.1016/j.toxlet.2018.02.025. ISSN 0378-4274. PMC 6549714. PMID 29481849.
- ↑ 44.0 44.1 44.2 Kaunelienė, Violeta; Meišutovič-Akhtarieva, Marija; Martuzevičius, Dainius (September 2018). "A review of the impacts of tobacco heating system on indoor air quality versus conventional pollution sources". Chemosphere. 206: 568–578. Bibcode:2018Chmsp.206..568K. doi:10.1016/j.chemosphere.2018.05.039. ISSN 0045-6535. PMID 29778082.
- ↑ 45.0 45.1 WHO 2020, p. 2.
- ↑ 46.0 46.1 McNeill 2018, p. 208.
- ↑ McNeill 2018, p. 210.
- ↑ 48.00 48.01 48.02 48.03 48.04 48.05 48.06 48.07 48.08 48.09 48.10 Fried, Nicholas D.; Gardner, Jason D. (2020). "Heat-not-burn tobacco products: an emerging threat to cardiovascular health". American Journal of Physiology-Heart and Circulatory Physiology. 319 (6): H1234–H1239. doi:10.1152/ajpheart.00708.2020. ISSN 0363-6135. PMC 7792702. PMID 33006919.
- ↑ Chaitanya Thandra, Krishna; Barsouk, Adam; Saginala, Kalyan; Sukumar Aluru, John; Barsouk, Alexander (February 2021). "Epidemiology of lung cancer". Współczesna Onkologia. 25 (1): 45–52. doi:10.5114/wo.2021.103829. PMC 8063897. PMID 33911981.
- ↑ Mravec, Boris; Tibensky, Miroslav; Horvathova, Lubica; Babal, Pavel (2020). "E-Cigarettes and Cancer Risk". Cancer Prevention Research. 13 (2): 137–144. doi:10.1158/1940-6207.CAPR-19-0346. ISSN 1940-6207. PMID 31619443.
- ↑ 51.0 51.1 51.2 Bracken-Clarke, Dara; Kapoor, Dhruv; Baird, Anne Marie; Buchanan, Paul James; Gately, Kathy; Cuffe, Sinead; Finn, Stephen P. (2021). "Vaping and lung cancer – A review of current data and recommendations". Lung Cancer. 153: 11–20. doi:10.1016/j.lungcan.2020.12.030. ISSN 0169-5002. PMID 33429159.
- ↑ Bafunno, Daniela; Catino, Annamaria; Lamorgese, Vito; Del Bene, Gabriella; Longo, Vito; Montrone, Michele; Pesola, Francesco; Pizzutilo, Pamela; Cassiano, Sandro; Mastrandrea, Angelica; Ricci, Donata; Petrillo, Patrizia; Varesano, Niccolò; Zacheo, Antonella; Galetta, Domenico (2020). "Impact of tobacco control interventions on smoking initiation, cessation, and prevalence: a systematic review". Journal of Thoracic Disease. 12 (7): 3844–3856. doi:10.21037/jtd.2020.02.23. ISSN 2072-1439. PMC 7399441. PMID 32802466.
- ↑ Tattan-Birch, Harry; Hartmann-Boyce, Jamie; Kock, Loren; Simonavicius, Erikas; Brose, Leonie; Jackson, Sarah; Shahab, Lion; Brown, Jamie (6 January 2022). "Heated tobacco products for smoking cessation and reducing smoking prevalence". The Cochrane database of systematic reviews. Wiley. 2022 (3). doi:10.1002/14651858.CD013790.pub2. ISSN 1465-1858. PMC 8733777. PMID 34988969.
- ↑ Mulier, Thomas; Chambers, Sam; Liefgreen, Liefgreen (24 March 2016). "Marlboro Kicks Some Ash". Bloomberg News.
- ↑ 55.0 55.1 Lasseter, Tom; Wilson, Duff; Wilson, Thomas; Bansal, Paritosh (15 May 2018). "Philip Morris device knows a lot about your smoking habit". Reuters.
- ↑ 56.0 56.1 56.2 56.3 56.4 56.5 Ziedonis, Douglas; Das, Smita; Larkin, Celine (2017). "Tobacco use disorder and treatment: New challenges and opportunities". Dialogues in Clinical Neuroscience. 19 (3): 271–80. doi:10.31887/DCNS.2017.19.3/dziedonis. PMC 5741110. PMID 29302224.
- ↑ 57.0 57.1 57.2 57.3 57.4 57.5 Luca, Alina-Costina; Curpăn, Alexandrina-Ștefania; Iordache, Alin-Constantin; Mîndru, Dana Elena; Țarcă, Elena; Luca, Florin-Alexandru; Pădureț, Ioana-Alexandra (8 February 2023). "Cardiotoxicity of Electronic Cigarettes and Heat-Not-Burn Tobacco Products—A Problem for the Modern Pediatric Cardiologist". Healthcare. 11 (4): 491. doi:10.3390/healthcare11040491. PMC 9957306. PMID 36833024.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Alina-Costina Luca, Alexandrina-Ștefania Curpăn, Alin-Constantin Iordache, Dana Elena Mîndru, Elena Țarcă, Florin-Alexandru Luca, Ioana-Alexandra Pădureț available under the CC BY 4.0 license.
This article incorporates text by Alina-Costina Luca, Alexandrina-Ștefania Curpăn, Alin-Constantin Iordache, Dana Elena Mîndru, Elena Țarcă, Florin-Alexandru Luca, Ioana-Alexandra Pădureț available under the CC BY 4.0 license.
- ↑ Cetinkaya, Pelin Duru; Pazarli Bostan, Pinar; Salepci, Banu; Gorekdilektasli, Asli; Elbek, Osman; Uyanusta Kucuk, Filiz Cagla; Karadogan, Dilek; Arpaz, Seren; Dulger, Seyhan; Uysal, Mehmet Atilla; Uzaslan, Esra; Ozge, Cengiz; Kilinc, Oguz; Dagli, Elif; Itil, Oya (18 July 2022). "Turkish Thoracic Society's Statement Report on Electronic Cigarettes and Heated Tobacco Products". Turkish Thoracic Journal. 23 (4): 296–301. doi:10.5152/TurkThoracJ.2022.22018}. PMC 9361150. PMID 35848438.
- ↑ Sund, Lachlan J.; Dargan, Paul I.; Archer, John R. H.; Wood, David M. (1 February 2023). "E-cigarette or vaping-associated lung injury (EVALI): a review of international case reports from outside the United States of America". Clinical Toxicology. 61 (2): 91–97. doi:10.1080/15563650.2022.2160342. PMID 36636876.
- ↑ WHO 2020, pp. 1–2.
- ↑ 61.0 61.1 61.2 61.3 61.4 61.5 61.6 Liu, Xiaoqiu; Lugo, Alessandra; Spizzichino, Lorenzo; Tabuchi, Takahiro; Gorini, Giuseppe; Gallus, Silvano (2018). "Heat-Not-Burn Tobacco Products Are Getting Hot in Italy". Journal of Epidemiology. 28 (5): 274–275. doi:10.2188/jea.JE20180040. ISSN 0917-5040. PMC 5911679. PMID 29657258.
 This article incorporates text by Xiaoqiu Liu, Alessandra Lugo, Lorenzo Spizzichino, Takahiro Tabuchi, Giuseppe Gorini, and Silvano Gallus available under the CC BY 4.0 license.
This article incorporates text by Xiaoqiu Liu, Alessandra Lugo, Lorenzo Spizzichino, Takahiro Tabuchi, Giuseppe Gorini, and Silvano Gallus available under the CC BY 4.0 license.
- ↑ England, Lucinda J.; Kim, Shin Y.; Tomar, Scott L; Ray, Cecily S; Gupta, Prakash C.; Eissenberg, Thomas; Cnattingius, Sven; Bernert, John T.; Tita, Alan Thevenet N.; Winn, Deborah M.; Djordjevic, Mirjana V.; Lambe, Mats; Stamilio, David; Chipato, Tsungai; Tolosa, Jorge E. (2010). "Non-cigarette tobacco use among women and adverse pregnancy outcomes". Acta Obstetricia et Gynecologica Scandinavica. 89 (4): 454–464. doi:10.3109/00016341003605719. ISSN 0001-6349. PMC 5881107. PMID 20225987.
- ↑ 63.0 63.1 63.2 63.3 63.4 63.5 63.6 Kotz, Daniel; Kastaun, Sabrina (2018). "E-Zigaretten und Tabakerhitzer: repräsentative Daten zu Konsumverhalten und assoziierten Faktoren in der deutschen Bevölkerung (die DEBRA-Studie)" [E-cigarettes and heat-not-burn products: representative data on consumer behaviour and associated factors in the German population (the DEBRA study)]. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (in Deutsch). 61 (11): 1407–1414. doi:10.1007/s00103-018-2827-7. ISSN 1436-9990. PMID 30284626. S2CID 52913095.
- ↑ "Alternative tobacco products use and its impact on urologic health – Will the lesser evil still be evil? A commentary and review of literature". Central European Journal of Urology. 74 (2). May 2021. doi:10.5173/ceju.2021.0110.
- ↑ 65.0 65.1 Ratajczak, Aleksandra; Jankowski, Piotr; Strus, Piotr; Feleszko, Wojciech (2020). "Heat Not Burn Tobacco Product—A New Global Trend: Impact of Heat-Not-Burn Tobacco Products on Public Health, a Systematic Review". International Journal of Environmental Research and Public Health. 17 (2): 409. doi:10.3390/ijerph17020409. ISSN 1660-4601. PMC 7014072. PMID 31936252.
 This article incorporates text by Aleksandra Ratajczak, Piotr Jankowski, Piotr Strus, and Wojciech Feleszko available under the CC BY 4.0 license.
This article incorporates text by Aleksandra Ratajczak, Piotr Jankowski, Piotr Strus, and Wojciech Feleszko available under the CC BY 4.0 license.
- ↑ Karanjkar, Rijula R.; Preshaw, Philip M.; Ellis, Janice S.; Holliday, Richard (February 2023). "Effect of tobacco and nicotine in causing staining of dental hard tissues and dental materials: A systematic review and meta‐analysis". Clinical and Experimental Dental Research. 9 (1): 150–164. doi:10.1002/cre2.683. PMC 9932248. PMID 36372903.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Rijula R Karanjkar, Philip M Preshaw, Janice S Ellis, and Richard Holliday available under the CC BY 4.0 license.
This article incorporates text by Rijula R Karanjkar, Philip M Preshaw, Janice S Ellis, and Richard Holliday available under the CC BY 4.0 license.
- ↑ 67.0 67.1 67.2 67.3 MHNZ 2017, p. 5.
- ↑ "Addictive nicotine and harmful substances also present in heated tobacco". Netherlands National Institute for Public Health and the Environment. 15 May 2018.
- ↑ 69.0 69.1 "Statement on the toxicological evaluation of novel heat-not-burn tobacco product" (PDF). Committee on Toxicity. 11 December 2017. pp. 1–10.
- ↑ 70.0 70.1 Tabuchi, Takahiro; Kiyohara, Kosuke; Hoshino, Takahiro; Bekki, Kanae; Inaba, Yohei; Kunugita, Naoki (2016). "Awareness and use of electronic cigarettes and heat-not-burn tobacco products in Japan". Addiction. 111 (4): 706–713. doi:10.1111/add.13231. ISSN 0965-2140. PMID 26566956.
- ↑ 71.0 71.1 Sun, Tianze; Anandan, Aathavan; Lim, Carmen C. W.; East, Katherine; Xu, Steve S.; Quah, Anne C. K.; Rutherford, Brienna N.; Johnson, Benjamin; Qi, Yaqi; Stjepanovic, Daniel; Leung, Janni; Connor, Jason P.; Gartner, Coral; Hall, Wayne D.; Vu, Giang; Chan, Gary C. K. (August 2023). "Global prevalence of heated tobacco product use, 2015–22: A systematic review and meta‐analysis". Addiction. 118 (8): 1430–1444. doi:10.1111/add.16199. PMID 37005862.
- ↑ 72.0 72.1 "What Do We Know About Heated Tobacco Products?". Centers for Disease Control and Prevention. 23 April 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 73.0 73.1 73.2 73.3 73.4 "Introduction to Heated Tobacco Products" (PDF). California Tobacco Control Program. California Department of Public Health. 2020. pp. 1–2.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Youth and Tobacco Use". Centers for Disease Control and Prevention. 10 November 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 75.0 75.1 75.2 75.3 "Heated Tobacco Products: Philip Morris International's IQOS" (PDF). Campaign for Tobacco-Free Kids. 9 February 2022. pp. 1–13.
- ↑ Forster, Mark; Liu, Chuan; Duke, Martin G; McAdam, Kevin G; Proctor, Christopher J (2015). "An experimental method to study emissions from heated tobacco between 100-200°C". Chemistry Central Journal. 9 (1): 20. doi:10.1186/s13065-015-0096-1. ISSN 1752-153X. PMC 4418098. PMID 25941536.
- ↑ 77.0 77.1 77.2 77.3 77.4 77.5 77.6 77.7 St.Helen, Gideon; Jacob III, Peyton; Nardone, Natalie; Benowitz, Neal L (2018). "IQOS: examination of Philip Morris International's claim of reduced exposure". Tobacco Control. 27 (Suppl 1): s30–s36. doi:10.1136/tobaccocontrol-2018-054321. ISSN 0964-4563. PMC 6252487. PMID 30158205.
 This article incorporates text by Gideon St.Helen, Peyton Jacob III, Natalie Nardone, and Neal L Benowitz available under the CC BY 4.0 license.
This article incorporates text by Gideon St.Helen, Peyton Jacob III, Natalie Nardone, and Neal L Benowitz available under the CC BY 4.0 license.
- ↑ Davis, Barbara; Williams, Monique; Talbot, Prue (20 February 2018). "iQOS: evidence of pyrolysis and release of a toxicant from plastic". Tobacco Control. 28 (1): tobaccocontrol–2017–054104. doi:10.1136/tobaccocontrol-2017-054104. ISSN 0964-4563. PMID 29535257. S2CID 3874502.
- ↑ 79.0 79.1 79.2 Lopez, Alexa A.; Hiler, Marzena; Maloney, Sarah; Eissenberg, Thomas; Breland, Alison B. (2016). "Expanding clinical laboratory tobacco product evaluation methods to loose-leaf tobacco vaporizers". Drug and Alcohol Dependence. 169: 33–40. doi:10.1016/j.drugalcdep.2016.10.005. ISSN 0376-8716. PMC 5140724. PMID 27768968.
- ↑ Chin, Neo Chai (2 March 2017). "Heated tobacco products just as bad as cigarettes: Amy Khor". Today (Singapore newspaper). Mediacorp.
- ↑ Adriaens, Karolien; Gucht, Dinska Van; Baeyens, Frank (2018). "IQOSTM vs. e-Cigarette vs. Tobacco Cigarette: A Direct Comparison of Short-Term Effects after Overnight-Abstinence". International Journal of Environmental Research and Public Health. 15 (12): 2902. doi:10.3390/ijerph15122902. ISSN 1660-4601. PMC 6313326. PMID 30567400.
 This article incorporates text by Karolien Adriaens, Dinska Van Gucht, and Frank Baeyens available under the CC BY 4.0 license.
This article incorporates text by Karolien Adriaens, Dinska Van Gucht, and Frank Baeyens available under the CC BY 4.0 license.
- ↑ Elias, Jesse; Ling, Pamela M (2018). "Invisible smoke: third-party endorsement and the resurrection of heat-not-burn tobacco products". Tobacco Control. 27 (Suppl 1): s96–s101. doi:10.1136/tobaccocontrol-2018-054433. ISSN 0964-4563. PMC 6238082. PMID 29875153.
- ↑ 83.0 83.1 Haig, Matt (2003). Brand Failures: The Truth about the 100 Biggest Branding Mistakes of All Time. Kogan Page Publishers. ISBN 978-0-7494-4433-4.
- ↑ Parker-Pope, Tara (10 February 2001). ""Safer" Cigarettes: A History". PBS.
- ↑ McGill, Douglas C (19 November 1988). "'Smokeless' Cigarette's Hapless Start". The New York Times. ISSN 0362-4331.
- ↑ 86.0 86.1 Hilts, Philip J. (27 November 1994). "Little Smoke, Little Tar, but Full Dose of Nicotine". The New York Times. ISSN 0362-4331.
- ↑ 87.0 87.1 O'Connell, Dominic (30 November 2016). "Philip Morris could stop making conventional cigarettes". BBC News.
- ↑ 88.0 88.1 88.2 Anderson, S J; Ling, P M (2008). ""And they told two friends...and so on": RJ Reynolds' viral marketing of Eclipse and its potential to mislead the public". Tobacco Control. 17 (4): 222–229. doi:10.1136/tc.2007.024273. ISSN 0964-4563. PMC 2845302. PMID 18332064.
- ↑ Haig, Matt (2005). Brand Failures: The Truth about the 100 Biggest Branding Mistakes of All Time. Kogan Page Publishers. pp. 51–. ISBN 978-0-7494-4433-4.
- ↑ Fisher, Daniel (29 May 2014). "Is This The Cigarette Of The Future, And Will The FDA Let You Buy It?". Forbes.
- ↑ "New heat-not-burn brand from RAI". Tobacco Journal International. 5 January 2015.
- ↑ 92.0 92.1 Craver, Richard (28 July 2015). "Reynolds ends Revo test market in Wisconsin". Winston-Salem Journal.
- ↑ "Reynolds launching heat-not-burn cigarette". CBS News. Associated Press. 14 November 2014.
- ↑ US 9486013, Sebastian, Andries Don; Billy Tyrone Conner & Chandra K. Banerjee et al., "Segmented smoking article with foamed insulation material", published 2016-11-08, assigned to R. J. Reynolds Tobacco Co.
- ↑ 95.00 95.01 95.02 95.03 95.04 95.05 95.06 95.07 95.08 95.09 95.10 95.11 95.12 95.13 95.14 95.15 Elias, Jesse; Dutra, Lauren M; St. Helen, Gideon; Ling, Pamela M (2018). "Revolution or redux? Assessing IQOS through a precursor product". Tobacco Control. 27 (Suppl 1): s102–s110. doi:10.1136/tobaccocontrol-2018-054327. ISSN 0964-4563. PMC 6238084. PMID 30305324.
- ↑ Pollack, Juddan (27 October 1997). "Philip Morris tries smokeless Accord: tobacco marketer, cautious about brand, doing 'consumer research'". Ad Age.
- ↑ "Anti-smoking body attacks smokeless cigarette device". Tobacco Journal International. 11 December 2007.
- ↑ Houston, Cameron (27 June 2007). "Revealed: tobacco giant's secret new weapon in the age of smoking bans". The Age.
- ↑ Lubin, Gus (25 June 2012). "Philip Morris Is Releasing A Bunch Of Crazy New Cigarettes". Business Insider.
- ↑ Cooper, Ted (1 February 2014). "Why Philip Morris International's New Heated Products Will Do Better Than Its Last Attempt". The Motley Fool.
- ↑ MacGuill, Shane (23 January 2014). "Has Philip Morris Learned from Heat-not-Burn Tobacco's Past?". Euromonitor International.
- ↑ Goldenson, Nicholas I.; Kirkpatrick, Matthew G.; Barrington-Trimis, Jessica L.; Pang, Raina D.; McBeth, Julia F.; Pentz, Mary Ann; Samet, Jonathan M.; Leventhal, Adam M. (2016). "Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology". Drug and Alcohol Dependence. 168: 176–180. doi:10.1016/j.drugalcdep.2016.09.014. ISSN 0376-8716. PMID 27676583.
- ↑ Davies, Rob; Monaghan, Angela (30 November 2016). "Philip Morris's vision of cigarette-free future met with scepticism". The Guardian.
- ↑ 104.0 104.1 104.2 104.3 104.4 Harlay, Jérôme (9 November 2016). "What you need to know about Heat-not-Burn (HNB) cigarettes". VapingPost.
- ↑ 105.0 105.1 "喜科推出入门级低温本草制品,6款口味已投放市场" [Cigoo introduces entry-level cryogenic herb products, and 6 flavors have been put on the market]. 蓝洞新消费 (in Chinese). 17 September 2020.
{{cite news}}: CS1 maint: unrecognized language (link) - ↑ WO application 2019179532, Cao, Yinghui; Tao Wang & Songfeng Wang et al., "Integrally formed heat-not-burn smoking product and preparation method therefor", published 2019-09-26, assigned to Yunnan Xike Technology Co. Ltd.
- ↑ 107.0 107.1 107.2 107.3 Staal, Yvonne CM; van de Nobelen, Suzanne; Havermans, Anne; Talhout, Reinskje (2018). "New Tobacco and Tobacco-Related Products: Early Detection of Product Development, Marketing Strategies, and Consumer Interest". JMIR Public Health and Surveillance. 4 (2): e55. doi:10.2196/publichealth.7359. ISSN 2369-2960. PMC 5996176. PMID 29807884.
 This article incorporates text by Yvonne CM Staal, Suzanne van de Nobelen, Anne Havermans, and Reinskje Talhout available under the CC BY 4.0 license.
This article incorporates text by Yvonne CM Staal, Suzanne van de Nobelen, Anne Havermans, and Reinskje Talhout available under the CC BY 4.0 license.
- ↑ 108.0 108.1 Esquire Editors (11 May 2019). "10 Best Weed Vaporizers to Buy in 2019". Esquire.
{{cite web}}: CS1 maint: uses authors parameter (link) - ↑ Crist, Ry (20 April 2019). "Weed tech heats up with a new smart vaporizer from Apple, Microsoft alums". CNET.
- ↑ "BAT finds strong Japan demand for its Glo smokeless tobacco device". The Japan Times. Reuters. 22 March 2017.
- ↑ 111.0 111.1 Caruana, Diane (25 October 2017). "BAT to launch its HnB device in Russia". VapingPost.
- ↑ 112.0 112.1 Caruana, Diane (25 September 2018). "BAT Launches Glo in Ukraine". VapingPost.
- ↑ Caplinger, Dan (31 May 2017). "Here's Why the Worst Might Be Yet to Come for Philip Morris International". The Motley Fool.
- ↑ "Innovation Drives BAT's $47 Billion Bid -- WSJ". ADVFN. 24 October 2016.
- ↑ 115.0 115.1 News Desk (24 October 2016). "World's second largest tobacco company tells people to quit smoking". The Express Tribune.
- ↑ Felberbaum, Michael (26 June 2014). "Philip Morris Int'l to Sell Marlboro HeatSticks". Salon. Associated Press. Archived from the original on 28 June 2014.
- ↑ Caplinger, Dan (23 November 2015). "5 Things Every Philip Morris Investor Should Know". The Motley Fool.
- ↑ Gillette, Felix; Kaplan, Jennifer; Chambers, Sam (8 March 2017). "Big Tobacco Has Caught Startup Fever". Bloomberg News.
- ↑ 119.0 119.1 Olivier, Wurlod (15 July 2017). "Une enquête de Reuters fait tousser Philip Morris" [Reuters investigation makes Philip Morris cough]. Tribune de Genève (in français).
- ↑ Nathan, Ralph (12 October 2016). "Why Philip Morris's iQOS Sales in Japan Are Promising". Market Realist.
- ↑ Schott, Paul (14 November 2021). "Will Philip Morris fulfill pledge to be 'majority smoke-free' in Connecticut?". CT Insider.
- ↑ Mulier, Thomas; Thesing, Gabi (26 June 2014). "Philip Morris Sees $700 Million Boost From iQOS Smoking Device". Bloomberg News.
- ↑ Uranaka, Taiga; Ando, Ritsuko (22 October 2018). "Philip Morris Aims to Revive Japan Sales With Cheaper Heat-Not-Burn Tobacco". U.S. News & World Report.
- ↑ LaVito, Angelica (22 October 2018). "Philip Morris unveils new smokeless cigarettes in a bid to rev up sales". CNBC.
- ↑ Hyo-sik, Lee (17 May 2017). "Philip Morris unveils smoke-free cigarette in Korea". The Korea Times.
- ↑ McKelvey, Karma; Popova, Lucy; Kim, Minji; Chaffee, Benjamin W; Vijayaraghavan, Maya; Ling, Pamela; Halpern-Felsher, Bonnie (November 2018). "Heated tobacco products likely appeal to adolescents and young adults". Tobacco Control. 27 (Suppl 1): s41–s47. doi:10.1136/tobaccocontrol-2018-054596. ISSN 0964-4563. PMC 6252490. PMID 30352843.
- ↑ 127.0 127.1 127.2 127.3 127.4 Auer, Reto; Concha-Lozano, Nicolas; Jacot-Sadowski, Isabelle; Cornuz, Jacques; Berthet, Aurélie (1 July 2017). "Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name". JAMA Internal Medicine. 177 (7): 1050–1052. doi:10.1001/jamainternmed.2017.1419. ISSN 2168-6106. PMC 5543320. PMID 28531246.
- ↑ Duprey, Rich (9 December 2017). "Will 2018 Be Philip Morris International Inc's Best Year Yet?". Billings Gazette.
- ↑ Rossel, Stefanie (1 June 2016). "All eyes on iQOS". Tobacco Reporter.
- ↑ 130.0 130.1 Tai, Mariko (31 August 2015). "Philip Morris rolls out iQOS smokeless smokes". Nikkei Asian Review.
- ↑ Kopa, Paulina Natalia; Pawliczak, Rafał (2019). "IQOS – a heat-not-burn (HnB) tobacco product – chemical composition and possible impact on oxidative stress and inflammatory response. A Systematic Review". Toxicology Mechanisms and Methods. 30 (2): 81–87. doi:10.1080/15376516.2019.1669245. ISSN 1537-6516. PMID 31532297. S2CID 202673535.
- ↑ 132.0 132.1 Drope, Jeffrey; Cahn, Zachary; Kennedy, Rosemary; Liber, Alex C.; Stoklosa, Michal; Henson, Rosemarie; Douglas, Clifford E.; Drope, Jacqui (2017). "Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine". CA: A Cancer Journal for Clinicians. 67 (6): 449–471. doi:10.3322/caac.21413. ISSN 0007-9235. PMID 28961314. S2CID 32928770.
- ↑ Caplinger, Dan (26 May 2017). "The FDA Moves Forward With Philip Morris iQOS Review". The Motley Fool.
- ↑ Lasseter, Tom; Bansal, Paritosh; Wilson, Thomas; Miyazaki, Ami; Wilson, Duff; Kalra, Aditya (20 December 2017). "Scientists describe problems in Philip Morris e-cigarette experiments". Reuters.
- ↑ 135.0 135.1 "FDA Panel Gives Qualified Support To Claims For". National Public Radio. 25 January 2018.
- ↑ 136.0 136.1 "FDA permits sale of IQOS Tobacco Heating System through premarket tobacco product application pathway". Food and Drug Administration. 30 April 2019.
- ↑ 137.0 137.1 LaVito, Angelica (4 October 2019). "Altria launches Iqos tobacco device in US, and the timing couldn't be better". CNBC.
- ↑ 138.0 138.1 138.2 138.3 138.4 "FDA Authorizes Marketing of IQOS Tobacco Heating System with 'Reduced Exposure' Information". United States Food and Drug Administration. 7 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 139.0 139.1 139.2 Lucas, Amelia (30 September 2021). "Philip Morris, Altria banned from importing or selling Iqos tobacco device in the U.S." CNBC.
- ↑ Maloney, Jennifer (29 September 2021). "U.S. Trade Body Rules Against Import of IQOS Heat-Not-Burn Tobacco Devices". Wall Street Journal.
- ↑ 141.0 141.1 "Consumer-Centric Vaping". Convenience Store Decisions. Harbor Communications. 4 March 2015.
- ↑ CSD Staff (4 March 2015). "Consumer-Centric Vaping". Convenience Store Decisions. Harbor Communications.
- ↑ "The iSmoke OneHitter, iSmoke "3-in-1" and iSmoke Oven join portfolio". Convenience Store News. 2017.
- ↑ 144.0 144.1 144.2 144.3 Schmid, Thomas (6 March 2019). "Heat-Not-Burn: Back to Tobacco". Tobacco Asia.
- ↑ 145.0 145.1 Song Seung-hyun (7 November 2017). "KT&G launches e-cigarette brand to take on foreign rivals". The Investor.
- ↑ "KT&G launches sales of new tobacco-heating device". Yonhap News Agency. 11 November 2017.
- ↑ 147.0 147.1 Jun, Sul-li (25 June 2021). "KT&G expands global foothold with 4,700 e-cigarette IP rights". Korea Economic Daily.
- ↑ 148.0 148.1 148.2 "New HNB Device from China Tobacco". Tobacco Asia. 30 May 2019.
- ↑ Biggs, John (17 June 2012). "Smoke Up: An Interview With The Creator Of The Ultracool Pax Vaporizer". TechCrunch.
- ↑ Lavrinc, Damon (1 July 2013). "Review: Ploom Model Two". Wired.
- ↑ Lawler, Ryan (11 March 2015). "The Pax 2 Improves Upon One Of The Best Vaporizers On The Market". TechCrunch.
- ↑ Yakowicz, Will (10 June 2015). "This Silicon Valley Company Just Raised $47 Million to Smoke Cigarette Makers". Inc.
- ↑ Adam Clark Estes (23 July 2015). "Pax 2 Vaporizer Review: It's Like Smoking In the Future". Gizmodo.
- ↑ 154.0 154.1 Stenovec, Tim (12 March 2016). "How two guys from Stanford built the 'iPhone of vaporizers'". Business Insider.
- ↑ Marinova, Polina (22 April 2019). "Vaping Startup Pax Labs Raises $420 Million in Funding: Term Sheet for Monday, April 22". Fortune.
- ↑ Taylor, Chris (19 June 2018). "Want to microdose marijuana? This company is making it easier for you". Mashable.
- ↑ 157.0 157.1 speccomm (26 August 2016). "Japan taking to heat-not-burn". Tobacco Reporter.
- ↑ Tuinstra, Taco (26 January 2016). "JT announces launch of next-generation Ploom". Tobacco Reporter.
- ↑ Uranaka, Taiga (16 January 2019). "Japan Tobacco ratchets up smokeless war with new products". Reuters.
- ↑ "JT to expand Ploom TECH sales". Tobacco Reporter. 10 October 2016.
- ↑ Uranaka, Taiga; Sarkar, Himani (29 May 2017). "Japan Tobacco plans to quadruple smokeless tobacco output capacity by 2018: CEO". Channel NewsAsia. Reuters.
- ↑ "Imperial Brands aims to convince smokers to switch to vaping products". Sky News. Yahoo! News. 6 November 2018.
- ↑ 163.0 163.1 Caruana, Diane (26 May 2021). "Imperial Brands Expresses Optimism About Its Heated Tobacco Device Pulze". VapingPost.
- ↑ 164.0 164.1 Kaplan, Jennifer (19 April 2018). "Philip Morris Plunges the Most in a Decade on Slump in Cigarettes". Bloomberg News.
- ↑ Stefanie Rossel (1 June 2017). "The heat is on". Tobacco Reporter.
- ↑ 166.00 166.01 166.02 166.03 166.04 166.05 166.06 166.07 166.08 166.09 166.10 Shi, Yuyan; Caputi, Theodore L.; Leas, Eric; Dredze, Mark; Cohen, Joanna E.; Ayers, John W. (2017). "They're heating up: Internet search query trends reveal significant public interest in heat-not-burn tobacco products". PLOS ONE. 12 (10): e0185735. Bibcode:2017PLoSO..1285735C. doi:10.1371/journal.pone.0185735. ISSN 1932-6203. PMC 5636077. PMID 29020019.
 This article incorporates text by Theodore L. Caputi, Eric Leas, Mark Dredze, Joanna E. Cohen, and John W. Ayers available under the CC BY 4.0 license.
This article incorporates text by Theodore L. Caputi, Eric Leas, Mark Dredze, Joanna E. Cohen, and John W. Ayers available under the CC BY 4.0 license.
- ↑ Ling, Pamela M.; Kim, Minji; Egbe, Catherine O.; Patanavanich, Roengrudee; Pinho, Mariana; Hendlin, Yogi (1 March 2022). "Moving targets: how the rapidly changing tobacco and nicotine landscape creates advertising and promotion policy challenges". Tobacco Control. 31 (2): 222–228. doi:10.1136/tobaccocontrol-2021-056552. PMID 35241592.
- ↑ Tabuchi, Takahiro; Shinozaki, Tomohiro; Kunugita, Naoki; Nakamura, Masakazu; Tsuji, Ichiro (2018). "Study Profile: The Japan "Society and New Tobacco" Internet Survey (JASTIS): A longitudinal internet cohort study of heat-not-burn tobacco products, electronic cigarettes and conventional tobacco products in Japan". Journal of Epidemiology. 29 (11): 444–450. doi:10.2188/jea.JE20180116. ISSN 0917-5040. PMC 6776477. PMID 30318495.
- ↑ Trefis Team (22 December 2017). "Increase In Heat-Not-Burn Tobacco Taxes In Japan To Hamper Growth". Nasdaq.
- ↑ speccomm (28 May 2019). "Heating up". Tobacco Reporter.
- ↑ Du, Lisa; Takahashi, Maiko; Cislo, Connor (21 November 2017). "Big Tobacco Faces Tax Reckoning in Japan on Hot New Devices". Boloomberg News.
- ↑ 172.0 172.1 "British American Tobacco hit by heated-tobacco slowdown as Japan growth stalls". The Japan Times. Bloomberg News. 13 June 2018.
- ↑ 173.0 173.1 "Tobacco company charged over importing prohibited product". The New Zealand Herald. 18 May 2017.
- ↑ Auer, Reto; Cornuz, Jacques; Berthet, Aurélie (2017). "Perplexing Conclusions Concerning Heat-Not-Burn Tobacco Cigarettes—Reply" (PDF). JAMA Internal Medicine. 177 (11): 1699–1700. doi:10.1001/jamainternmed.2017.5861. ISSN 2168-6106. PMID 29114801.
- ↑ 175.0 175.1 Jane Cheung (24 May 2019). "New forms of smoking catch on". The Standard.
- ↑ 176.00 176.01 176.02 176.03 176.04 176.05 176.06 176.07 176.08 176.09 176.10 Berg, Carla J.; Bar-Zeev, Yael; Levine, Hagai (2020). "Informing iQOS Regulations in the United States: A Synthesis of What We Know". SAGE Open. 10 (1): 215824401989882. doi:10.1177/2158244019898823. ISSN 2158-2440. PMC 7384757. PMID 32719733.
 This article incorporates text by Carla J. Berg, Yael Bar-Zeev, and Hagai Levine2 available under the CC BY 4.0 license.
This article incorporates text by Carla J. Berg, Yael Bar-Zeev, and Hagai Levine2 available under the CC BY 4.0 license.
- ↑ 177.0 177.1 177.2 Mathers, Annalise; Schwartz, Robert; O'Connor, Shawn; Fung, Michael; Diemert, Lori (2018). "Marketing IQOS in a dark market". Tobacco Control. 28 (2): tobaccocontrol–2017–054216. doi:10.1136/tobaccocontrol-2017-054216. ISSN 0964-4563. PMID 29724866. S2CID 19103708.
- ↑ 178.0 178.1 Katz, MH; Redberg, RF (6 November 2017). "Science Requires Open Discourse". JAMA Internal Medicine. 178 (1): 15–16. doi:10.1001/jamainternmed.2017.5763. PMID 29114738.
- ↑ 179.0 179.1 179.2 Leas, Eric C; Cohen, Joanna E; Ayers, John W (2020). "A Philip Morris advertisement for its heated tobacco product IQOS sets a troubling precedent". Tobacco Control: tobaccocontrol-2019-055363. doi:10.1136/tobaccocontrol-2019-055363. ISSN 0964-4563.
- ↑ Levy, David T.; Cummings, K. Michael; Villanti, Andrea C.; Niaura, Ray; Abrams, David B.; Fong, Geoffrey T.; Borland, Ron (2017). "A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products". Addiction. 112 (1): 8–17. doi:10.1111/add.13394. ISSN 0965-2140. PMC 5079857. PMID 27109256.
- ↑ 181.0 181.1 181.2 181.3 181.4 Kirkham, Chris (10 May 2019). "Exclusive: Philip Morris suspends social media campaign after Reuters exposes young 'influencers'". Reuters.
- ↑ Myroniuk, Anna (25 February 2020). "Investigation: IQOS uses Instagram influencers to target youth in Ukraine". Kyiv Post.
- ↑ Kirkham, Chris (21 February 2020). "Inside the Philip Morris campaign to "normalize" a tobacco device". Reuters.
- ↑ Watts, Christina; Burton, Suzan; Freeman, Becky (15 November 2020). "Creating a market for IQOS: analysis of Philip Morris' strategy to introduce heated tobacco products to the Australian consumer market". Tobacco control. BMJ: tobaccocontrol–2020–056057. doi:10.1136/tobaccocontrol-2020-056057. ISSN 0964-4563. PMID 33191270.
- ↑ Kim, Samuel CJ; Friedman, Theodore C (January 2022). "A New Ingenious Enemy: Heat-Not-Burn Products". Tobacco Use Insights. 15: 1179173X2210764. doi:10.1177/1179173X221076419. PMC 8883376. PMID 35237081.
- ↑ 186.0 186.1 Lempert, Lauren Kass; Glantz, Stanton A (2018). "Heated tobacco product regulation under US law and the FCTC". Tobacco Control. 27 (Suppl 1): s118–s125. doi:10.1136/tobaccocontrol-2018-054560. ISSN 0964-4563. PMC 6204223. PMID 30291201.
- ↑ 187.0 187.1 WHO 2020, p. 3.
- ↑ "Heated Tobacco Products: Global regulation" (PDF). Campaign for Tobacco-Free Kids. September 2020. pp. 1–4.
- ↑ Liber, Alex C. (2018). "Heated tobacco products and combusted cigarettes: comparing global prices and taxes". Tobacco Control. 28 (6): tobaccocontrol–2018–054602. doi:10.1136/tobaccocontrol-2018-054602. ISSN 0964-4563. PMID 30381439. S2CID 53173353.
- ↑ 190.0 190.1 Glantz, Stanton A (2018). "Heated tobacco products: the example of IQOS". Tobacco Control. 27 (Suppl 1): s1–s6. doi:10.1136/tobaccocontrol-2018-054601. ISSN 0964-4563. PMC 6252052. PMID 30352841.
- ↑ Fisher, Daniel (16 June 2014). "Philip Morris International Bets Big On The Future Of Smoking". Forbes.
- ↑ McNeill 2018, p. 201.
- ↑ Siegel-Itzkovich, Judy (2 March 2017). "Orgs slam Litzman for allowing sale of iQOS heated, smokeless cigarettes". The Jerusalem Post.
- ↑ Ronny Linder-Ganz (16 January 2018). "In Blow to Philip Morris, Israel to Tax iQOS E-cigarettes Like Ordinary Cigarettes". Haaretz.
- ↑ Judy Siegel-Itzkovich (3 April 2018). "Justice Ministry says iQOS product will be treated as ordinary tobacco". The Jerusalem Post.
- ↑ 196.0 196.1 Yui, Monami (28 August 2016). "Big Tobacco Wants to Turn Japan's Smokers Into Vapers". Bloomberg News.
- ↑ "Tax hike on heat-not-burn tobacco products under consideration as LDP begins review of tax reforms". The Japan Times. 8 September 2017.
- ↑ Jae-hyuk, Park (26 May 2017). "IQOS available in Seoul Saturday". The Korea Times.
- ↑ 199.0 199.1 Trefis Team (13 September 2017). "Why Is Korea Easier To Conquer For iQOS Than Europe?". Nasdaq.
- ↑ "Singapore Enhances Tobacco Control Measures". Ministry of Health (Singapore). 28 July 2016. Archived from the original on 31 July 2017.
- ↑ Ho, Sai Yin; Chen, Jianjiu; Leung, Lok Tung; Mok, Hoi Yan; Wang, Lijun; Wang, Man Ping; Lam, Tai Hing (2019). "Adolescent Smoking in Hong Kong: Prevalence, Psychosocial Correlates, and Prevention". Journal of Adolescent Health. 64 (6): S19–S27. doi:10.1016/j.jadohealth.2019.01.003. ISSN 1054-139X. PMID 31122545.
- ↑ 202.0 202.1 "Tobacco Control Legislation". 30 April 2022.
- ↑ Elder, Vaughan (13 January 2017). "Legality of tobacco product in question". Otago Daily Times.
- ↑ Caruana, Diane (3 February 2017). "iQos heatsticks declared illegal in NZ". VapingPost.
- ↑ 205.0 205.1 "New Zealand's legal action against IQOS postponed, consultation with Big Tobacco follows". 130 (1465). New Zealand Medical Journal. 10 November 2017.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "iQOS may not be as harm-free as claimed, study finds". The New Zealand Herald. 14 March 2018.
- ↑ Zaharia, Marius (27 March 2018). "New Zealand court gives Philip Morris nod to sell heated tobacco product". Reuters.
- ↑ MHNZ 2017, p. ii.
- ↑ de Granda-Orive, José Ignacio; Solano-Reina, Segismundo; Jiménez-Ruiz, Carlos A. (May 2022). "Tobacco as a Source of Microplastics. Tobacco and Environment: World No Tobacco Day 2022". Archivos de Bronconeumología. 58 (5): 395–397. doi:10.1016/j.arbres.2022.04.005. PMID 35570088.
- ↑ Leigh, Noel J; Tran, Phillip L; O’Connor, Richard J; Goniewicz, Maciej Lukasz (2018). "Cytotoxic effects of heated tobacco products (HTP) on human bronchial epithelial cells". Tobacco Control. 27 (Suppl 1): s26–s29. doi:10.1136/tobaccocontrol-2018-054317. ISSN 0964-4563. PMC 6252481. PMID 30185530.
- ↑ Kary, Tiffany (18 April 2019). "Philip Morris says it doesn't want you to buy its cigarettes, but will IQOS help it survive?". The Japan Times. Bloomberg News.
- ↑ Belushkin, M.; Esposito, M.; Jaccard, G.; Jeannet, C.; Korneliou, A.; Tafin Djoko, D. (2018). "Role of testing standards in smoke-free product assessments". Regulatory Toxicology and Pharmacology. 98: 1–8. doi:10.1016/j.yrtph.2018.06.021. ISSN 0273-2300. PMID 29983383.
- ↑ Kamada, Takahiro; Yamashita, Yosuke; Tomioka, Hiromi (2016). "Acute eosinophilic pneumonia following heat-not-burn cigarette smoking". Respirology Case Reports. 4 (6): e00190. doi:10.1002/rcr2.190. ISSN 2051-3380. PMC 5167280. PMID 28031826.
- ↑ Meg Neal (2 January 2015). "What Is a Heat-Not-Burn Cigarette and Can It Help You Quit?". Gizmodo.
- ↑ 215.0 215.1 215.2 Mallock, Nadja; Böss, Lisa; Burk, Robert; Danziger, Martin; Welsch, Tanja; Hahn, Harald; Trieu, Hai-Linh; Hahn, Jürgen; Pieper, Elke; Henkler-Stephani, Frank; Hutzler, Christoph; Luch, Andreas (2018). "Levels of selected analytes in the emissions of "heat not burn" tobacco products that are relevant to assess human health risks". Archives of Toxicology. 92 (6): 2145–2149. doi:10.1007/s00204-018-2215-y. ISSN 0340-5761. PMC 6002459. PMID 29730817.
- ↑ Paumgartten, Francisco J.R. (2018). "A critical appraisal of the harm reduction argument for heat-not-burn tobacco products". Revista Panamericana de Salud Pública. 42: e161. doi:10.26633/RPSP.2018.161. ISSN 1020-4989. PMC 6386018. PMID 31093189.
- ↑ Jenssen, Brian P.; Boykan, Rachel (2019). "Electronic Cigarettes and Youth in the United States: A Call to Action (at the Local, National and Global Levels)". Children. 6 (2): 30. doi:10.3390/children6020030. ISSN 2227-9067. PMC 6406299. PMID 30791645.
- ↑ Craver, Richard (2 August 2015). "Reynolds' decision to stop marketing of heated cigarette Revo illustrates challenges in selling adult smokers on new products". Winston-Salem Journal.
- ↑ Jeong, Won Tae; Cho, Hyun Ki; Lee, Hyung Ryeol; Song, Ki Hoon; Lim, Heung Bin (2019). "Comparison of the content of tobacco alkaloids and tobacco-specific nitrosamines in 'heat-not-burn' tobacco products before and after aerosol generation". Inhalation Toxicology. 30 (13–14): 527–533. doi:10.1080/08958378.2019.1572840. ISSN 0895-8378. PMID 30741569.
- ↑ Emma, Rosalia; Caruso, Massimo; Campagna, Davide; Pulvirenti, Roberta; Li Volti, Giovanni (September 2022). "The Impact of Tobacco Cigarettes, Vaping Products and Tobacco Heating Products on Oxidative Stress". Antioxidants. 11 (9): 1829. doi:10.3390/antiox11091829.
- ↑ Alarabi, Ahmed B.; Lozano, Patricia A.; Khasawneh, Fadi T.; Alshbool, Fatima Z. (February 2022). "The effect of emerging tobacco related products and their toxic constituents on thrombosis". Life Sciences. 290: 120255. doi:10.1016/j.lfs.2021.120255.
- ↑ 222.0 222.1 Hendlin, Yogi Hale; Elias, Jesse; Ling, Pamela M. (2017). "The Pharmaceuticalization of the Tobacco Industry". Annals of Internal Medicine. 167 (4): 278–280. doi:10.7326/M17-0759. ISSN 0003-4819. PMC 5568794. PMID 28715843.
- ↑ Rossel, Stefanie (1 July 2016). "Blending nature and technology". Tobacco Reporter.
- ↑ Besaratinia, Ahmad; Tommasi, Stella (2020). "Vaping epidemic: challenges and opportunities". Cancer Causes & Control. 31 (7): 663–667. doi:10.1007/s10552-020-01307-y. ISSN 0957-5243. PMC 7274878. PMID 32363571.
- ↑ Pacitto, A.; Stabile, L.; Scungio, M.; Rizza, V.; Buonanno, G. (2018). "Characterization of airborne particles emitted by an electrically heated tobacco smoking system". Environmental Pollution. 240: 248–254. doi:10.1016/j.envpol.2018.04.137. ISSN 0269-7491. PMID 29747109.
- ↑ Ye, Dongxia; Rahman, Irfan (10 February 2023). "Emerging Oral Nicotine Products and Periodontal Diseases". International Journal of Dentistry. 2023: 1–7. doi:10.1155/2023/9437475. PMC 9937772. PMID 36819641.
{{cite journal}}: Check|pmc=value (help) - ↑ Gaca, Marianna; Williamson, Justine; Digard, Helena; Adams, Louise; Hawkridge, Lauren; Proctor, Christopher (6 August 2022). "Bridging: Accelerating Regulatory Acceptance of Reduced-Risk Tobacco and Nicotine Products". Nicotine & Tobacco Research. 24 (9): 1371–1378. doi:10.1093/ntr/ntac041. PMC 9356683. PMID 35171296.
- ↑ McNeill 2018, p. 30.
- ↑ "Philip Morris' Smokeless Tobacco Stick Shouldn't Be Marketed As Safer Than Cigarettes, FDA Panel Says". Kaiser Health News. 26 January 2018.
- ↑ Queloz, Sébastien; Etter, Jean-François (2019). "An online survey of users of tobacco vaporizers, reasons and modes of utilization, perceived advantages and perceived risks". BMC Public Health. 19 (1): 642. doi:10.1186/s12889-019-6957-0. ISSN 1471-2458. PMC 6537171. PMID 31133009.
 This article incorporates text by Sébastien Queloz and Jean-François Etter available under the CC BY 4.0 license.
This article incorporates text by Sébastien Queloz and Jean-François Etter available under the CC BY 4.0 license.
- ↑ Unger, Michael; Unger, Darian W. (2018). "E-cigarettes/electronic nicotine delivery systems: a word of caution on health and new product development". Journal of Thoracic Disease. 10 (S22): S2588–S2592. doi:10.21037/jtd.2018.07.99. ISSN 2072-1439. PMC 6178300. PMID 30345095.