Benazepril
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /bəˈnæzəprɪl/ |
| Trade names | Lotensin, others |
| |
| Clinical data | |
| Drug class | ACE inhibitor[1] |
| Main uses | High blood pressure, heart failure, diabetic kidney disease[1] |
| Side effects | Feeling tired, dizziness, cough, light-headedness with standing[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 7.5 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692011 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | 96.7% |
| Metabolism | Liver glucuronidation |
| Elimination half-life | 10 hours[3] |
| Excretion | Kidney and biliary |
| Chemical and physical data | |
| Formula | C24H28N2O5 |
| Molar mass | 424.497 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Benazepril, sold under the brand name Lotensin among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] It is taken by mouth.[1] Versions are available as the combinations benazepril/hydrochlorothiazide and benazepril/amlodipine.[1]
Common side effects include feeling tired, dizziness, cough, and light-headedness with standing.[1] Serious side effects may include kidney problems, low blood pressure, high blood potassium, and angioedema.[1] Use in pregnancy may harm the baby while use when breastfeeding maybe okay.[4] It is an ACE inhibitor and works by decreasing renin-angiotensin-aldosterone system activity.[1]
Benazepril was patented in 1981 and came into medical use in 1990.[5] It is available as a generic medication.[1] A month supply in the United States costs about US$1.32 as of 2019.[6] In 2017, it was the 104th most commonly prescribed medication in the United States, with more than seven million prescriptions.[7][8]
Medical uses
It is useful for high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] Other reasonable initial options include angiotensin II receptor antagonists, calcium-channel blockers, and thiazide diuretics.[1]
Dosage
The defined daily dose is 7.5 mg by mouth.[2]
Side effects
The most common side effects patients experience are a headache or a chronic cough. The chronic cough develops in about 20% of patients treated,[9] and those patients that experience it find it develops after a few months of use. Anaphylaxis, angioedema, and elevation of potassium levels are more serious side effects that can also occur.
Contraindications
Benazepril should be discontinued during pregnancy, as it can harm the fetus.
Society and culture
Cost
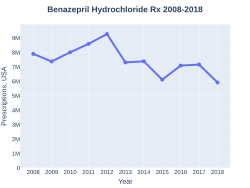
A month supply in the United States costs about US$1.32 as of 2019.[6] In 2017, it was the 104th most commonly prescribed medication in the United States, with more than seven million prescriptions.[7][8]
-
BenazeprilHydrochloride costs (US)
-
BenazeprilHydrochloride prescriptions (US)
Dosage forms
It is also available in combination with hydrochlorothiazide, under the trade name Lotensin HCT', and with amlodipine (trade name Lotrel).
Veterinary use
Under the brand names Fortekor (Novartis) and VetACE (Jurox Animal Health),[citation needed] benazepril hydrochloride is used to treat congestive heart failure in dogs[10][11] and chronic kidney failure in cats and dogs.[citation needed]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Benazepril Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 4 August 2020. Retrieved 3 March 2019.
- ↑ 2.0 2.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 25 November 2020. Retrieved 6 September 2020.
- ↑ Benowitz, Neal L. (2020). "11. Antihypertensive agents". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 183. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-12-05.
- ↑ "Benazepril Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 29 November 2020. Retrieved 3 March 2019.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 468. ISBN 9783527607495. Archived from the original on 2019-03-01. Retrieved 2019-03-01.
- ↑ 6.0 6.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ 7.0 7.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 8.0 8.1 "Benazepril Hydrochloride - Drug Usage Statistics". ClinCalc. Archived from the original on 3 March 2021. Retrieved 11 April 2020.
- ↑ Dykewicz, Mark S. (April 2004). "Cough and Angioedema From Angiotensin-Converting Enzyme Inhibitors: New Insights Into Mechanisms and Management". Medscape. Archived from the original on 26 February 2021. Retrieved 2 April 2014.
- ↑ King JN, Mauron C, Kaiser G (December 1995). "Pharmacokinetics of the active metabolite of benazepril, benazeprilat, and inhibition of plasma angiotensin-converting enzyme activity after single and repeated administrations to dogs". Am. J. Vet. Res. 56 (12): 1620–8. PMID 8599524.
- ↑ O'Grady MR, O'Sullivan ML, Minors SL, Horne R (2009). "Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers". J. Vet. Intern. Med. 23 (5): 977–83. doi:10.1111/j.1939-1676.2009.0346.x. PMID 19572914.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- All articles with unsourced statements
- Articles with unsourced statements from November 2013
- Articles with invalid date parameter in template
- ACE inhibitors
- Acetic acids
- Benzazepines
- Enantiopure drugs
- Ethyl esters
- Lactams
- Prodrugs
- Butyrates
- Novartis brands
- RTT