Fenoprofen
 | |
 | |
| Names | |
|---|---|
| Trade names | Nalfon, others |
| |
| Clinical data | |
| Drug class | Nonsteroidal anti-inflammatory drug (NSAID)[1] |
| Main uses | Pain inflammation[1] |
| Side effects | Heart burn, nausea, headache, dizziness, anxiety, swelling[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Onset of action | Within 30 min[1] |
| Duration of action | Up to 6 hrs[1] |
| Typical dose | 200 to 600 mg QID[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681026 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Metabolism | Major urinary metabolites are fenoprofen glucuronide and 4′-hydroxyfenoprofen glucuronide. |
| Elimination half-life | 3 hours |
| Excretion | Kidney (~90%) |
| Chemical and physical data | |
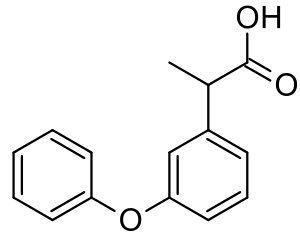
| Formula | C15H14O3 |
| Molar mass | 242.274 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fenoprofen, sold under the brand name Nalfon among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat mild to moderate pain and inflammation.[1] This may include in rheumatoid arthritis, gout, and osteoarthritis.[1] It is taken by mouth.[1] Onsets is within 30 minutes and last up to 6 hours.[1]
Common side effects include heart burn, nausea, headache, dizziness, anxiety, and swelling.[1] Other side effects may include liver problems, Stevens-Johnson syndrome, anaphylaxis, kidney problems, heart failure, stomach bleeding, heart attack, and high blood pressure.[1] Use in the later part of pregnancy may harm the baby.[1] It works by blocking COX1 and COX2.[1]
Fenoprofen was approved for medical use in the United States in 1976.[1] It is available as a generic medication.[2] In the United States 30 tablets of 600 mg cost about 30 USD as of 2021.[2] It is not available in the United Kingdom as of 2021.[3]
Medical uses
Dosage
It is taken at a dose of 200 mg to 600 mg three to four times per day.[1] The maximum dose is 3,200 mg a day.[1]
Contraindications
History of significantly impaired renal function; patients with known hypersensitivity to any component of the product; patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs; treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery.
Side effects
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[4][5] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[4][5]
Interactions
- Aminoglycosides (e.g. gentamicin): Plasma aminoglycoside levels may be elevated.
- Angiotensin-converting enzyme (ACE) inhibitors: Antihypertensive effect of ACE inhibitors may be diminished.
- Anticoagulants: Coadministration may prolong prothrombin time.
- Aspirin: Fenoprofen Cl may be increased; coadministration is not recommended.
- Diuretics: Patients treated with fenoprofen may be resistant to the effects of loop diuretics and thiazides.
- Hydantoins, sulfonamides, sulfonylureas: Fenoprofen may displace these drugs from their binding site.
- Lithium: Renal Cl of lithium may be reduced and plasma levels may be elevated, which may increase the risk of lithium toxicity.
- Methotrexate: May increase methotrexate levels.
- Phenobarbital: May decrease fenoprofen t ½ . Dosage adjustments of fenoprofen may be required if phenobarbital is added or withdrawn.
- SSRIs (e.g. fluoxetine, citalopram): The risk of GI effects may be increased.
Laboratory test
False elevation in free and total serum T3 as measured by Amerlex-M kit.
Pharmacology
Decreases inflammation, pain, and fever, probably through inhibition of cyclooxygenase (COX-2 inhibitor) activity and prostaglandin synthesis.
Society and culture
Cost
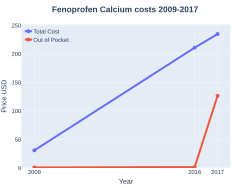
The cost of this medication in the U.S. for $290 (USD) for 90 capsules (400 mg) [6]
-
Fenoprofen costs (US)
-
Fenoprofen prescriptions (US)
Brand names
- UK - Fenopron (Typharm Limited)
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 "Fenoprofen Monograph for Professionals". Drugs.com. Archived from the original on 20 January 2021. Retrieved 10 December 2021.
- ↑ 2.0 2.1 "Fenoprofen Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 10 December 2021.
- ↑ "Fenoprofen tablets for pain and inflammation". patient.info. Archived from the original on 19 October 2021. Retrieved 10 December 2021.
- ↑ 4.0 4.1 "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Archived from the original on 16 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 5.0 5.1 "NSAIDs may cause rare kidney problems in unborn babies". U.S. Food and Drug Administration. 21 July 2017. Archived from the original on 17 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Fenoprofen Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 January 2021. Retrieved 30 March 2021.
External links
| Identifiers: |
|
|---|
- Fenoprofen info from Drugs.com Archived 2016-03-03 at the Wayback Machine
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- Webarchive template wayback links
- Propionic acids
- Diphenyl ethers
- Nonsteroidal anti-inflammatory drugs
- RTT