Delafloxacin
 | |
| Names | |
|---|---|
| Trade names | Baxdela, Quofenix |
| Other names | Delafloxacin meglumine; ABT-492; RX-3341; WQ-3034 |
| |
| Clinical data | |
| Drug class | Fluoroquinolone[1] |
| Main uses | Bacterial skin and skin structure infections[2] |
| Side effects | Diarrhea, nausea, tendinitis, peripheral neuropathy, psychosis, Clostridioides difficile infection[1] |
| WHO AWaRe | |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
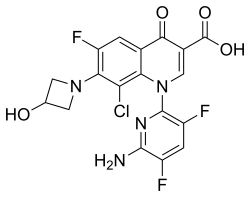
| Formula | C18H12ClF3N4O4 |
| Molar mass | 440.76 g·mol−1 |
| 3D model (JSmol) | |
| |
Delafloxacin sold under the brand name Baxdela among others, is an antibiotic used to treat bacterial skin and skin structure infections.[2] It is generally effective for MRSA and Pseudomonas.[1] It may be given by mouth or by injection into a vein.[2]
Common side effects include diarrhea and nausea.[2] Other side effects may include tendinitis, peripheral neuropathy, psychosis, Clostridioides difficile infection, and anaphylaxis.[1] Safety in pregnancy is unclear.[1] It is a fluoroquinolone.[1]
Delafloxacin was approved for medical use in the United States in 2017 and Europe in 2019.[1][2] In the United Kingdom five days of treatment costs the NHS about £615 as of 2021.[3] This amount in the United States is about 760 USD.[4]
Medical use
Delafloxacin is indicated to treat adults with acute bacterial skin and skin structure infections (ABSSSI) caused by designated susceptible bacteria or adults with community-acquired bacterial pneumonia (CABP) caused by designated susceptible bacteria.[5]
Susceptible bacteria for ABSSSI are:[5]
- Gram-positive organisms: Staphylococcus aureus (including methicillin-resistant [MRSA] and methicillin-susceptible [MSSA] isolates), Staphylococcus haemolyticus, Staphylococcus lugdunensis, Streptococcus agalactiae, Streptococcus anginosus group (including Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus), Streptococcus pyogenes, and Enterococcus faecalis
- Gram-negative organisms: Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Susceptible bacteria for CABP are:[5] Streptococcus pneumoniae, Staphylococcus aureus (methicillin-susceptible [MSSA] isolates only), Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Haemophilus influenzae, Haemophilus parainfluenzae, Chlamydia pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae.
It has not been tested in pregnant women.[5]
In the European Union it is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in adults when it is considered inappropriate to use other antibacterial agents that are commonly recommended for the initial treatment of these infections.[2]
Dosage
It is typically used at a dose of 300 mg intravenous or 450 mg by mouth twice per day.[2] The duration of treatment is 5 days to two weeks.[2]
Side effects
Like other drugs in the fluoroquinolone class, delafloxacin contains a black box warning about the risk of tendinitis, tendon rupture, peripheral neuropathy, central nervous system effects, and exacerbation of myasthenia gravis. The label also warns against the risk of hypersensitivity reactions and Clostridium difficile-associated diarrhea.[5]
Side effects occurring in more than 2% of clinical trial subjects included nausea, diarrhea, headache, elevated transaminases, and vomiting.[5]
Interactions
Like other fluoroquinolones, delafloxacin chelates metals including aluminum, magnesium, sucralfate, iron, zinc, and divalent and trivalent cations like didanosine; using this drugs with antacids, some dietary supplements, or drugs buffered with any of these ions will interfere with available amounts of delafloxacin.[5]
Pharmacology
The half-life varies in around 8 hours at normal doses. Excretion is 65% through urine, mostly in unmetabolized form, and 28% via feces. Clearance is reduced in people with severe kidney disease.[6]
Delafloxacin is more active (lower MIC90) than other quinolones against Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). In contrast to most approved fluoroquinolones, which are zwitterionic, delafloxacin has an anionic character, which results in a 10-fold increase in delafloxacin accumulation in both bacteria and cells at acidic pH. This property is believed to confer to delafloxacin an advantage for the eradication of Staphylococcus aureus in acidic environments, including intracellular infections and biofilms.[6]
Chemistry
The chemical name is 1-deoxy-1 (methylamino)-D-glucitol, 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (salt).[5]
The injectable form of delafloxacin is sold as the meglumine salt of the active ingredient and its United States Adopted Name, delafloxacin meglumine, reflects that; the injection formulation also includes EDTA and sulfobutylether-β-cyclodextrin. The tablet is made of delafloxacin, citric acid anhydrous, crospovidone, magnesium stearate, microcrystalline cellulose, povidone, sodium bicarbonate, and sodium phosphate monobasic monohydrate.[5]
History

Delafloxacin was known as ABT-492, RX-3341, and WQ-3034 while it was under development.[8]
Rib-X Pharmaceuticals acquired delafloxacin from Wakunaga Pharmaceutical in 2006.[9] Rib-X was renamed to Melinta Therapeutics in 2013.[10] It was developed and marketed by Melinta Therapeutics (formerly Rib-X Pharmaceuticals),[5] which subsequently merged with Cempra.[11]
Key clinical trials for delafloxacin have been performed by Melinta regarding indications for skin and skin structure infections as well as complicated bacterial infections and uncomplicated gonorrhea. The trial on gonorrhea was terminated before data was released.[7]
Delafloxacin was approved by the FDA in June 2017, after it was noninferior to vancomycin plus aztreonam in two trials on 1042 patients with acute bacterial skin and skin structure infection.[12] New Drug Applications (NDA) for delafloxacin (Baxdela) 450 mg tablets and 300 mg injections were approved by the FDA in June 2017.[13]
The FDA obligated Melinta to conduct further studies as follows:[13]
- a 5-year surveillance study to determine if resistance emerges, with the final report due in December 2022
- a study of the IV form in pregnant rats to determine distribution to the reproductive tract, due June 2018, with further studies required if there is significant distribution.
Melinta merged with Cempra in August, 2017.[11]
Melinta has entered into commercialization and distribution agreements with both Menarini Therapeutics (March 2017) and Eurofarma Laboratórios (January 2015) for international commercialization of delafloxacin. The agreement with Menarini allows them to commercialize and distribute in 68 countries, including Europe, China, and South Korea among others. A similar agreement with Eurofarma allows for commercialization in Brazil.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Delafloxacin Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 22 December 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Quofenix EPAR". European Medicines Agency (EMA). 16 October 2019. Archived from the original on 5 August 2020. Retrieved 12 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 595. ISBN 978-0857114105.
- ↑ "Baxdela Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 22 December 2021.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 5.9 "Baxdela- delafloxacin meglumine tablet Baxdela- delafloxacin meglumine injection, powder, lyophilized, for solution". DailyMed. 12 February 2020. Archived from the original on 28 November 2020. Retrieved 12 May 2020.
- ↑ 6.0 6.1 Candel, FJ; Peñuelas, M (2017). "Delafloxacin: design, development and potential place in therapy". Drug Design, Development and Therapy. 11: 881–891. doi:10.2147/DDDT.S106071. PMC 5367733. PMID 28356714.
- ↑ 7.0 7.1 7.2 Markham A (September 2017). "Delafloxacin: First Global Approval". Drugs. 77 (13): 1481–1486. doi:10.1007/s40265-017-0790-5. PMC 6208769. PMID 28748399.
- ↑ "Delafloxacin". AdisInsight. Archived from the original on 16 July 2017. Retrieved 10 July 2017.
- ↑ Cartwright, Heather (12 July 2011). "Rib-X Pharmaceuticals Signs Global Antibiotic Research Collaboration with Sanofi". PharmaDeals Review. No. 7. doi:10.3833/pdr.v2011i7.1494 (inactive 31 May 2021). Archived from the original on 25 April 2012.
{{cite news}}: CS1 maint: DOI inactive as of May 2021 (link) - ↑ Stearns, John (August 1, 2016). "Melinta Therapeutics takes aim at deadly drug-resistant bacteria". Hartford Business Journal. Archived from the original on April 22, 2019. Retrieved June 4, 2021.
- ↑ 11.0 11.1 "Cempra Press Releases". Archived from the original on 2017-10-28. Retrieved 2021-06-04.
- ↑ Osborne, Randy (20 June 2017). "Melinta's I.V., oral delafloxacin wins FDA nod in skin infections". BioWorld. Archived from the original on 3 August 2019. Retrieved 4 June 2021.
- ↑ 13.0 13.1 "NDA Approval Letter: NDA 208610 and NDA 208611" (PDF). FDA. June 19, 2017. Archived (PDF) from the original on March 30, 2021. Retrieved June 4, 2021.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Delafloxacin meglumine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-06-10. Retrieved 2021-06-04.
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- CS1 maint: DOI inactive as of May 2021
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Azetidines
- Chloroarenes
- Fluoroquinolone antibiotics
- Pyridines
- Carboxylic acids
- RTT