Purine nucleoside phosphorylase deficiency
Purine nucleoside phosphorylase deficiency, is a rare autosomal recessive[1] metabolic disorder which results in immunodeficiency.
Signs and symptoms
In addition to the symptoms associated with immunodeficiency, such as depletion of T-cells, decline of lymphocyte activity, and an abrupt proliferation of both benign and opportunistic infections — PNP-deficiency is often characterized by the development of autoimmune disorders. lupus erythematosus, autoimmune hemolytic anemia, and idiopathic thrombocytopenic purpura have been reported with PNP-deficiency.[2] Neurological symptoms, such as developmental decline, hypotonia, and mental retardation have also been reported.[citation needed]
Cause
The disorder is caused by a mutation of the purine nucleoside phosphorylase (PNP) gene, located at chromosome 14q13.1.[3][4] This mutation was first identified by Eloise Giblett, a professor at the University of Washington, in 1975.[5] PNP is a key enzyme in the purine catabolic[6] pathway, and is required for purine degradation. Specifically, it catalyzes the conversion of inosine to hypoxanthine and guanosine to guanine (both guanine and hypoxanthine will be made into xanthine which will then become uric acid). A deficiency of it leads to buildup of elevated deoxy-GTP (dGTP) levels resulting in T-cell toxicity and deficiency.[4][7] In contrast to adenosine deaminase deficiency (another deficiency of purine metabolism), there is minimal disruption to B cells.[8]
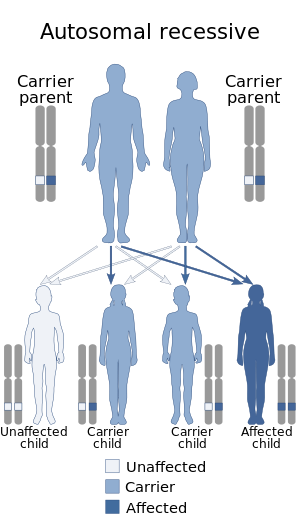
PNP deficiency is inherited in an autosomal recessive manner.[1] This means the defective gene responsible for the disorder is located on an autosome (chromosome 14 is an autosome), and two copies of the defective gene (one inherited from each parent) are required in order to be born with the disorder. The parents of an individual with an autosomal recessive disorder both carry one copy of the defective gene, but usually do not experience any signs or symptoms of the disorder.[citation needed]
Diagnosis
Diagnosis is based on the clinical examination and on laboratory findings showing leukopenia, severe lymphopenia with low CD3, CD4, and CD8 counts and variable B cell function and immunoglobulin levels. Neutropenia has also been reported. Hallmark diagnostic markers of PNP deficiency include hypouricemia, complete or near complete absence of PNP activity in red blood cell lysate and increased urine or blood levels of inosine, guanosine and their deoxy forms. Diagnosis is confirmed by genetic screening of PNP.[citation needed]
Differential diagnosis
Differential diagnosis includes aplastic anemias, SCID, severe combined immunodeficiency due to adenosine deaminase deficiency, ataxia-telangiectasia, and viral meningoencephalitis.[citation needed]
Screening
Measurement of T cell receptor excision circles during newborn screening for SCID can detect some patients suffering from PNP deficiency, although removal of metabolites by maternal PNP may delay the deleterious effects on PNP-deficient lymphocytes. Few newborn screening programs also measure purine metabolites in dried[citation needed]
Treatment
Supportive treatment, including intravenous immunoglobulin therapy, prophylaxis for Pneumocystis carinii, and physical, occupational, and speech therapy, reduces the risk of infection and may encourage optimal neurologic development for patients.[citation needed]
Epidemiology
PNP-deficiency is extremely rare. Only 33 patients with the disorder in the United States have been documented.[2] In the United Kingdom only two children have been diagnosed with this disorder in 1994 and 2008.[9]
See also
References
- ↑ 1.0 1.1 Sasaki Y, Iseki M, Yamaguchi S, Kurosawa Y, Yamamoto T, Moriwaki Y, Kenri T, Sasaki T, Yamashita R (July 1998). "Direct evidence of autosomal recessive inheritance of Arg24 to termination codon in purine nucleoside phosphorylase gene in a family with a severe combined immunodeficiency patient". Human Genetics. 103 (1): 81–85. doi:10.1007/s004390050787. PMID 9737781. S2CID 8373698.
- ↑ 2.0 2.1 Markert ML (1991). "Purine nucleoside phosphorylase deficiency". Immunodefic Rev. 3 (1): 45–81. PMID 1931007.
- ↑ Online Mendelian Inheritance in Man (OMIM): 164050
- ↑ 4.0 4.1 Snyder FF, Jenuth JP, Mably ER, Mangat RK (Mar 1997). "Point mutations at the purine nucleoside phosphorylase locus impair thymocyte differentiation in the mouse". Proc. Natl. Acad. Sci. U.S.A. 94 (6): 2522–2527. Bibcode:1997PNAS...94.2522S. doi:10.1073/pnas.94.6.2522. PMC 20121. PMID 9122228.
- ↑ Motulsky A, Gartler S. "Biographical Memoirs: Eloise R. Giblett". National Academy of Sciences.
- ↑ Berg, Jeremy M.; Tymoczko, John L.; Stryer, Lubert (2010-12-24). Biochemistry (7th ed.). p. 753. ISBN 9781429229364.
- ↑ Toro A, Grunebaum E (Oct 2006). "TAT-mediated intracellular delivery of purine nucleoside phosphorylase corrects its deficiency in mice". J. Clin. Invest. 116 (10): 2717–2726. doi:10.1172/JCI25052. PMC 1560347. PMID 16964310.
- ↑ "eMedicine - Purine Nucleoside Phosphorylase Deficiency : Article by Alan P Knutsen". Archived from the original on October 15, 2008. Retrieved July 25, 2010.
- ↑ "Archive copy". Archived from the original on 2009-03-11. Retrieved 2021-10-08.
{{cite web}}: CS1 maint: archived copy as title (link)
External links
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- CS1 maint: archived copy as title
- All articles with unsourced statements
- Articles with unsourced statements from October 2021
- Articles with invalid date parameter in template
- Articles with unsourced statements from September 2020
- Noninfectious immunodeficiency-related cutaneous conditions
- Autosomal recessive disorders
- Rare diseases
- Inborn errors of purine-pyrimidine metabolism
- Combined T and B–cell immunodeficiencies
