Oral submucous fibrosis
| Oral submucous fibrosis | |
|---|---|
| Other names: OSMF or OSF | |
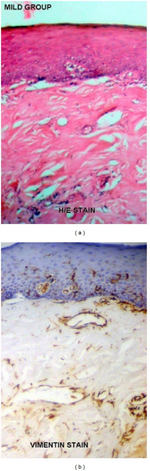 | |
| a) Oral submucous fibrosis under H/E staining b) oral submucous fibrosis under vimentin immunohistochemical staining | |
| Specialty | Oral medicine and Dentistry and Oral Pathology |
Oral submucous fibrosis is a chronic, complex, premalignant (1% transformation risk) condition of the oral cavity, characterized by juxta-epithelial inflammatory reaction and progressive fibrosis of the submucosal tissues (the lamina propria and deeper connective tissues). As the disease progresses, the oral mucosa becomes fibrotic to the point that the person is unable to open the mouth.[1][2] The condition is remotely linked to oral cancers and is associated with areca nut and / or its by-products chewing, majorly practiced in South and South-East Asian countries.[3] The incidence of OSMF has also increased in the western countries due to the changing habits and constant migrating population.[4]
Symptoms and signs
In the initial phase of the disease, the mucosa feels leathery with palpable fibrotic bands. In the advanced stage the oral mucosa loses its resiliency and becomes blanched and stiff. The disease is believed to begin in the posterior part of the oral cavity and gradually spread outward.
Other features of the disease include:
- Xerostomia
- Recurrent ulceration
- Pain in the ear or deafness
- Nasal intonation of voice
- Restriction of the movement of the soft palate
- A budlike shrunken uvula
- Thinning and stiffening of the lips
- Pigmentation of the oral mucosa
- Dryness of the mouth and burning sensation (stomatopyrosis)
- Decreased mouth opening and tongue protrusion
Causes
Dried products such as paan masala and gutkha have higher concentrations of areca nut and appear to cause the disease. Other causes include:
- Immunological diseases
- Extreme climatic conditions
- Prolonged deficiency to iron and vitamins in the diet
Pathogenesis
"Exposure to areca nut (Areca catechu) containing products with or without tobacco (ANCP/T) is currently believed to lead to OSF in individuals with genetic immunologic or nutritional predisposition to the disease."[5]
This hypersensitivity reaction results in a juxta-epithelial inflammation that leads to increased fibroblastic activity and decreased breakdown of fibers. The fibroblasts are phenotypically modified, and the fibers they form are more stable, produce thicker bundles that progressively become less elastic. once the original loosely arranged fibrous tissue is replaced by the ongoing fibrosis, the movability of the oral tissues is reduced, there is loss of flexibility and reduced opening of the mouth.
These collagen fibers are non degradable and the phagocytic activity is minimized.
According to a recent cross sectional study the time taken for return of salivary pH to baseline levels after chewing areca nut containing mixtures is significantly longer in habitual users with OSF when compared to unaffected users.[5] Prolonged Alkaline pH induces death fetal fibroblast type and replacement by a profibrotic fibroblast.[5] The patterns of intraoral fibrotic bands produced by alkaline chemical injury mimic those produced by areca nut chewing.[6] Sharma et al., have equated the pathogenesis of OSF to a over-healing wound, to explain its evolution as well as malignant transformation.[6][7] Increased mechanical stiffness through YAP/TAZ pathway accelerates the malignant transformation of OSF.[8] The atrophic epithelium in OSF has been attributed to the senescence of basal stem cell layer and the development of hyperplastic epithelium through senescence escape[7][9].
Diagnosis
Definitions
Per Jens J Pindborg and Satyavati Sirsat (1966) (Pathological definition)- ‘An insidious chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibro-elastic change of the lamina propria, with epithelial atrophy leading to stiffness.’[10]
Per Mohit Sharma and Raghu Radhakrishnan (2019) - 'An insidious, chronic potentially malignant fibrotic disorder affecting the entire oral cavity and sometimes the pharynx and oesophagus. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibroelastic change of the lamina propria with epithelial atrophy leading to stiffness of the oral mucosa, progressive decrement in mouth opening and inability to eat'[11]
Per Chandramani More and Naman Rao (2019) (Clinical definition)- ‘A debilitating, progressive, irreversible collagen metabolic disorder induced by chronic chewing of areca nut and its commercial preparations; affecting the oral mucosa and occasionally the pharynx and esophagus; leading to mucosal stiffness and functional morbidity; and has a potential risk of malignant transformation.’[12]
Classification
Oral submucous fibrosis is clinically divided into three stages:[13]
- Stage 1: Stomatitis
- Stage 2: Fibrosis
- a- Early lesions, blanching of the oral mucosa
- b- Older lesions, vertical and circular palpable fibrous bands in and around the mouth or lips, resulting in a mottled, marble-like appearance of the buccal mucosa
- Stage 3: Sequelae of oral submucous fibrosis
- a- Leukoplakia
- b- Speech and hearing deficits
Khanna and Andrade in 1995 developed a group classification system for the surgical management of trismus:[14]
- Group I: Earliest stage without mouth opening limitations with an interincisal distance of greater than 35 mm.
- Group II: Patients with an interincisal distance of 26–35 mm.
- Group III: Moderately advanced cases with an interincisal distance of 15–26 mm. Fibrotic bands are visible at the soft palate, and pterygomandibular raphe and anterior pillars of fauces are present.
- Group IVA: Trismus is severe, with an interincisal distance of less than 15 mm and extensive fibrosis of all the oral mucosa.
- Group IVB: Disease is most advanced, with premalignant and malignant changes throughout the mucosa.tumor necrosis factor alpha and keratin 17 are
interdependent regulators, they could be used as diagnostic makers and a prognostic mirror of oral submucous fibrosis cases[15]
Treatment
Biopsy screening although necessary is not mandatory most dentist can visually examine the area and proceed with the proper course of treatment.
Treatment includes:
- Abstention from chewing areca nut (also known as betel nut) and tobacco
- Minimizing consumption of spicy foods, including chiles
- Maintaining proper oral hygiene
- Supplementing the diet with foods rich in vitamins A, B complex, and C and iron
- Forgoing hot fluids like tea, coffee
- Forgoing alcohol
- Employing a dental surgeon to round off sharp teeth and extract third molars
- Interprofessional treatment approach [16]
Treatment also includes following:
- The prescription of chewable pellets of hydrocortisone (Efcorlin); one pellet to be chewed every three to four hours for three to four weeks
- 0.5 ml intralesional injection Hyaluronidase 1500 IU mixed in 1 ml of Lignocaine into each buccal mucosa once a week for 4 weeks or more as per condition
- 0.5 ml intralesional injection of Hyaluronidase 1500 IU and 0.5 ml of injection Hydrocortisone acetate 25 mg/ml in each buccal mucosa once a week alternatively for 4 weeks or more as per condition[17]
- Submucosal injections of hydrocortisone 100 mg once or twice daily depending upon the severity of the disease for two to three weeks
- Submucosal injections of human chorionic gonadotrophins (Placentrax) 2-3 ml per sitting twice or thrice in a week for three to four weeks
- Surgical treatment is recommended in cases of progressive fibrosis when interincisor distance becomes less than 2 centimetres (0.79 in). (Multiple release incisions deep to mucosa, submucosa and fibrotic tissue and suturing the gap or dehiscence so created by mucosal graft obtained from tongue and Z-plasty. In this procedure multiple deep z-shaped incisions are made into fibrotic tissue and then sutured in a straighter fashion.)
- Pentoxifylline (Trental), a methylxanthine derivative that has vasodilating properties and increases mucosal vascularity, is also recommended as an adjunct therapy in the routine management of oral submucous fibrosis.[18]
- IFN-gamma is antifibrotic cytokine which alters collagen synthesis and helps in OSF.[19]
- Colchicine tablets 0.5 mg twice a day[20]
- Lycopene, 16 mg a day helps in improvement of OSF[21]
The treatment of patients with oral submucous fibrosis depends on the degree of clinical involvement.[22] If the disease is detected at a very early stage, cessation of the habit is sufficient. Most patients with oral submucous fibrosis present with moderate-to-severe disease. Severe oral submucous fibrosis is irreversible. Moderate oral submucous fibrosis is reversible with cessation of habit and mouth opening exercise. Current modern day medical treatments can make the mouth opening to normal minimum levels of 30 mm mouth opening with proper treatment.
Epidemiology
The incidence of the disease is higher in people from certain parts of the world including South and South East Asian, South Africa and the Middle Eastern countries.[23]
Research
Recently scientists have proven that intralesional injection of autologous bone marrow stem cells is a safe and effective treatment modality in oral sub mucosal fibrosis. It has been shown autologous bone marrow stem cell injections induces angiogenesis in the area of lesion which in turn decreases the extent of fibrosis thereby leading to significant increase in mouth opening.[24][25]
History
In 1952, T.Sheikh coined the term distrophica idiopathica mucosa oris to describe an oral fibrosing disease he discovered in five Indian women from Kenya.[26] S.G. Joshi subsequently coined the termed oral submucous fibrosis (OSF) for the condition in 1953.[27]
See also
References
- ↑ Cox SC, Walker DM (October 1996). "Oral submucous fibrosis. A review". Australian Dental Journal. 41 (5): 294–9. doi:10.1111/j.1834-7819.1996.tb03136.x. PMID 8961601.
- ↑ Aziz SR (1997). "Oral submucous fibrosis: an unusual disease". Journal of the New Jersey Dental Association. 68 (2): 17–9. PMID 9540735.
- ↑ More CB, Rao NR, More S, Johnson NW (2020). "Reasons for Initiation of Areca Nut and Related Products in Patients with Oral Submucous Fibrosis within an Endemic Area in Gujarat, India". Substance Use & Misuse. 55 (9): 1413–1421. doi:10.1080/10826084.2019.1660678. PMID 32569538. S2CID 219991434.
- ↑ More C, Shah P, Rao N, Pawar R (2015). "Oral Submucous Fibrosis: An Overview with Evidence Based Management". International Journal of Oral Health Sciences and Advances. 3 (3): 40–9.
- ↑ 5.0 5.1 5.2 Donoghue M, Basandi PS, Adarsh H, Madhushankari GS, Selvamani M, Nayak P (2015). "Habit-associated salivary pH changes in oral submucous fibrosis-A controlled cross-sectional study". Journal of Oral and Maxillofacial Pathology. 19 (2): 175–81. doi:10.4103/0973-029X.164529. PMC 4611925. PMID 26604493.
- ↑ 6.0 6.1 Sharma M, Shetty SS, Radhakrishnan R (2018-07-31). "Oral Submucous Fibrosis as an Overhealing Wound: Implications in Malignant Transformation". Recent Patents on Anti-Cancer Drug Discovery. 13 (3): 272–291. doi:10.2174/1574892813666180227103147. PMID 29485009. Archived from the original on 2020-10-19. Retrieved 2022-04-18.
- ↑ 7.0 7.1 Sharma M, Fonseca FP, Hunter KD, Radhakrishnan R (August 2020). "Loss of oral mucosal stem cell markers in oral submucous fibrosis and their reactivation in malignant transformation". International Journal of Oral Science. 12 (1): 23. doi:10.1038/s41368-020-00090-5. PMC 7442837. PMID 32826859.
- ↑ Sharma, Mohit; Hunter, Keith D.; Fonseca, Felipe Paiva; Shetty, Smitha Sammith; Radhakrishnan, Raghu (August 2021). "Role of Yes-associated protein and transcriptional coactivator with PDZ-binding motif in the malignant transformation of oral submucous fibrosis". Archives of Oral Biology. 128: 105164. doi:10.1016/j.archoralbio.2021.105164. Archived from the original on 2022-04-21. Retrieved 2022-04-18.
- ↑ Sharma, Mohit; Hunter, Keith D.; Fonseca, Felipe Paiva; Radhakrishnan, Raghu (2021-10). "Emerging role of cellular senescence in the pathogenesis of oral submucous fibrosis and its malignant transformation". Head & Neck. 43 (10): 3153–3164. doi:10.1002/hed.26805. ISSN 1043-3074. Archived from the original on 2022-04-21. Retrieved 2022-04-18.
{{cite journal}}: Check date values in:|date=(help) - ↑ Pindborg JJ, Sirsat SM (December 1966). "Oral submucous fibrosis". Oral Surgery, Oral Medicine, and Oral Pathology. 22 (6): 764–79. doi:10.1016/0030-4220(66)90367-7. PMID 5224185.
- ↑ Sharma M, Radhakrishnan R (May 2019). "Revisiting and revising the definition of oral submucous fibrosis". Oral Oncology. 92: 94. doi:10.1016/j.oraloncology.2019.03.004. PMID 30853277. Archived from the original on 2022-04-21. Retrieved 2022-04-18.
- ↑ More CB, Rao NR (2019). "Proposed clinical definition for oral submucous fibrosis". Journal of Oral Biology and Craniofacial Research. 9 (4): 311–314. doi:10.1016/j.jobcr.2019.06.016. PMC 6614531. PMID 31334003.
- ↑ Pindborg JJ (September 1989). "Oral submucous fibrosis: a review". Annals of the Academy of Medicine, Singapore. 18 (5): 603–7. PMID 2694917.
- ↑ Khanna JN, Andrade NN (December 1995). "Oral submucous fibrosis: a new concept in surgical management. Report of 100 cases". International Journal of Oral and Maxillofacial Surgery. 24 (6): 433–9. doi:10.1016/S0901-5027(05)80473-4. PMID 8636640.
- ↑ Abd El Latif GA (January 2019). "Tumor necrosis factor alpha and keratin 17 expression in oral submucous fibrosis in rat model". Egyptian Dental Journal. 65 (1 (Oral Medicine, X–Ray, Oral Biology & Oral Pathology) pages - 277–88): 277–288. doi:10.21608/edj.2015.71414.
- ↑ Rao NR, Villa A, More CB, Jayasinghe RD, Kerr AR, Johnson NW (January 2020). "Oral submucous fibrosis: a contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management". Journal of Otolaryngology - Head & Neck Surgery. 49 (1): 3. doi:10.1186/s40463-020-0399-7. PMC 6951010. PMID 31915073.
- ↑ Kakar PK, Puri RK, Venkatachalam VP (January 1985). "Oral submucous fibrosis--treatment with hyalase". The Journal of Laryngology and Otology. 99 (1): 57–9. doi:10.1017/S0022215100096286. PMID 3968475.
- ↑ Rajendran R, Rani V, Shaikh S (2006). "Pentoxifylline therapy: a new adjunct in the treatment of oral submucous fibrosis". Indian Journal of Dental Research. 17 (4): 190–8. doi:10.4103/0970-9290.29865. PMID 17217216.
- ↑ Haque MF, Meghji S, Nazir R, Harris M (January 2001). "Interferon gamma (IFN-gamma) may reverse oral submucous fibrosis". Journal of Oral Pathology & Medicine. 30 (1): 12–21. doi:10.1034/j.1600-0714.2001.300103.x. PMID 11140895.
- ↑ Krishnamoorthy B, Khan M (July 2013). "Management of oral submucous fibrosis by two different drug regimens: A comparative study". Dental Research Journal. 10 (4): 527–32. PMC 3793419. PMID 24130591.
- ↑ Kumar A, Bagewadi A, Keluskar V, Singh M (February 2007). "Efficacy of lycopene in the management of oral submucous fibrosis". Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 103 (2): 207–13. doi:10.1016/j.tripleo.2006.07.011. PMID 17234537.
- ↑ More CB, Gavli N, Chen Y, Rao NR (2018). "A novel clinical protocol for therapeutic intervention in oral submucous fibrosis: An evidence based approach". Journal of Oral and Maxillofacial Pathology. 22 (3): 382–391. doi:10.4103/jomfp.JOMFP_223_18. PMC 6306594. PMID 30651684.
- ↑ Oral Submucous Fibrosis at eMedicine
- ↑ Sankaranarayanan S, Padmanaban J, Ramachandran CR, Manjunath S, Baskar S, Senthil Kumar R, Senthil Nagarajan R, Murugan P, Srinivasan V, Abraham S (June 2008). Autologous Bone Marrow stem cells for treatment of Oral Sub-Mucous Fibrosis - a case report. Sixth Annual Meeting of International Society for Stem Cell Research (ISSCR). Philadelphia. Archived from the original on 2016-03-17. Retrieved 2022-04-18.
- ↑ Abraham S, Sankaranarayanan S, Padmanaban J, Manimaran K, Srinivasan V, Senthil Nagarajan R, Murugan P, Manjunath S, Senthil Kumar R, Baskar S (June 2008). Autologous Bone Marrow Stem Cells in Oral Submucous Fibrosis – Our experience in three cases with six months follow-up. 8th Annual Meeting of Japanese Society of Regenerative Medicine. Vol. 68. Tokyo, Japan. pp. 233–55. Archived from the original on 2016-03-17. Retrieved 2022-04-18.
- ↑ Hetland G, Johnson E, Lyberg T, Bernardshaw S, Tryggestad AM, Grinde B (October 2008). "Effects of the medicinal mushroom Agaricus blazei Murill on immunity, infection and cancer". Scandinavian Journal of Immunology. 68 (4): 363–70. doi:10.1111/j.1365-3083.2008.02156.x. PMID 18782264.
- ↑ Joshi SG (1952). "Fibrosis of the palate and pillars". Indian Journal of Otolaryngology. 4 (1): 1–4.
External links
| Classification | |
|---|---|
| External resources |