Stroke
| Stroke | |
|---|---|
| Other names: Cerebrovascular accident (CVA), cerebrovascular insult (CVI), brain attack | |
 | |
| CT scan of the brain showing a prior right-sided ischemic stroke from blockage of an artery. Changes on a CT may not be visible early on.[1] | |
| Specialty | Neurology, stroke medicine |
| Symptoms | Inability to move or feel on one side of the body, problems understanding or speaking, dizziness, loss of vision to one side[2][3] |
| Complications | Persistent vegetative state[4] |
| Causes | Ischemic (blockage) and hemorrhagic (bleeding)[5] |
| Risk factors | High blood pressure, tobacco smoking, obesity, high blood cholesterol, diabetes mellitus, previous TIA, end-stage kidney disease, atrial fibrillation[2][6][7] |
| Diagnostic method | Based on symptoms with medical imaging typically used to rule out bleeding[8][9] |
| Differential diagnosis | Low blood sugar[8] |
| Treatment | Based on the type[2] |
| Prognosis | Average life expectancy 1 year[2] |
| Frequency | 42.4 million (2015)[10] |
| Deaths | 6.3 million (2015)[11] |
A stroke is a medical condition in which poor blood flow to the brain causes cell death.[5] There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding.[5] Both cause parts of the brain to stop functioning properly.[5] Signs and symptoms of a stroke may include an inability to move or feel on one side of the body, problems understanding or speaking, dizziness, or loss of vision to one side.[2][3] Signs and symptoms often appear soon after the stroke has occurred.[3] If symptoms last less than one or two hours, the stroke is a transient ischemic attack (TIA), also called a mini-stroke.[3] A hemorrhagic stroke may also be associated with a severe headache.[3] The symptoms of a stroke can be permanent.[5] Long-term complications may include pneumonia and loss of bladder control.[3]
The main risk factor for stroke is high blood pressure.[6] Other risk factors include tobacco smoking, obesity, high blood cholesterol, diabetes mellitus, a previous TIA, end-stage kidney disease, and atrial fibrillation.[2][6][7] An ischemic stroke is typically caused by blockage of a blood vessel, though there are also less common causes.[12][13][14] A hemorrhagic stroke is caused by either bleeding directly into the brain or into the space between the brain's membranes.[12][15] Bleeding may occur due to a ruptured brain aneurysm.[12] Diagnosis is typically based on a physical exam and supported by medical imaging such as a CT scan or MRI scan.[8] A CT scan can rule out bleeding, but may not necessarily rule out ischemia, which early on typically does not show up on a CT scan.[9] Other tests such as an electrocardiogram (ECG) and blood tests are done to determine risk factors and rule out other possible causes.[8] Low blood sugar may cause similar symptoms.[8]
Prevention includes decreasing risk factors, surgery to open up the arteries to the brain in those with problematic carotid narrowing, and warfarin or other anticoagulants in people with atrial fibrillation.[2] Aspirin or statins may be recommended for prevention.[2] A stroke or TIA often requires emergency care.[5] An ischemic stroke, if detected within three to four and half hours, may be treatable with a medication that can break down the clot.[2] Some hemorrhagic strokes benefit from surgery.[2] Treatment to attempt recovery of lost function is called stroke rehabilitation, and ideally takes place in a stroke unit; however, these are not available in much of the world.[2]
In 2013 approximately 6.9 million people had an ischemic stroke and 3.4 million people had a hemorrhagic stroke.[16] In 2015 there were about 42.4 million people who had previously had a stroke and were still alive.[10] Between 1990 and 2010 the number of strokes which occurred each year decreased by approximately 10% in the developed world and increased by 10% in the developing world.[17] In 2015, stroke was the second most frequent cause of death after coronary artery disease, accounting for 6.3 million deaths (11% of the total).[11] About 3.0 million deaths resulted from ischemic stroke while 3.3 million deaths resulted from hemorrhagic stroke.[11] About half of people who have had a stroke live less than one year.[2] Overall, two thirds of strokes occurred in those over 65 years old.[17]
Classification
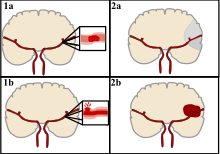

Strokes can be classified into two major categories: ischemic and hemorrhagic.[18] Ischemic strokes are caused by interruption of the blood supply to the brain, while hemorrhagic strokes result from the rupture of a blood vessel or an abnormal vascular structure. About 87% of strokes are ischemic, the rest being hemorrhagic. Bleeding can develop inside areas of ischemia, a condition known as "hemorrhagic transformation." It is unknown how many hemorrhagic strokes actually start as ischemic strokes.[2]
Definition
In the 1970s the World Health Organization defined stroke as a "neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours",[19] although the word "stroke" is centuries old. This definition was supposed to reflect the reversibility of tissue damage and was devised for the purpose, with the time frame of 24 hours being chosen arbitrarily. The 24-hour limit divides stroke from transient ischemic attack, which is a related syndrome of stroke symptoms that resolve completely within 24 hours.[2] With the availability of treatments that can reduce stroke severity when given early, many now prefer alternative terminology, such as brain attack and acute ischemic cerebrovascular syndrome (modeled after heart attack and acute coronary syndrome, respectively), to reflect the urgency of stroke symptoms and the need to act swiftly.[20]
Ischemic
In an ischemic stroke, blood supply to part of the brain is decreased, leading to dysfunction of the brain tissue in that area. There are four reasons why this might happen:
- Thrombosis (obstruction of a blood vessel by a blood clot forming locally)
- Embolism (obstruction due to an embolus from elsewhere in the body),[2]
- Systemic hypoperfusion (general decrease in blood supply, e.g., in shock)[21]
- Cerebral venous sinus thrombosis.[22]
A stroke without an obvious explanation is termed cryptogenic (of unknown origin); this constitutes 30–40% of all ischemic strokes.[2][23]
There are various classification systems for acute ischemic stroke. The Oxford Community Stroke Project classification (OCSP, also known as the Bamford or Oxford classification) relies primarily on the initial symptoms; based on the extent of the symptoms, the stroke episode is classified as total anterior circulation infarct (TACI), partial anterior circulation infarct (PACI), lacunar infarct (LACI) or posterior circulation infarct (POCI). These four entities predict the extent of the stroke, the area of the brain that is affected, the underlying cause, and the prognosis.[24][25] The TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification is based on clinical symptoms as well as results of further investigations; on this basis, a stroke is classified as being due to (1) thrombosis or embolism due to atherosclerosis of a large artery, (2) an embolism originating in the heart, (3) complete blockage of a small blood vessel, (4) other determined cause, (5) undetermined cause (two possible causes, no cause identified, or incomplete investigation).[26] Users of stimulants such as cocaine and methamphetamine are at a high risk for ischemic strokes.[27]
Hemorrhagic
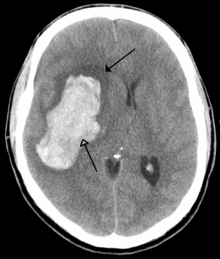
There are two main types of hemorrhagic stroke:[28][29]
- Intracerebral hemorrhage, which is basically bleeding within the brain itself (when an artery in the brain bursts, flooding the surrounding tissue with blood), due to either intraparenchymal hemorrhage (bleeding within the brain tissue) or intraventricular hemorrhage (bleeding within the brain's ventricular system).
- Subarachnoid hemorrhage, which is basically bleeding that occurs outside of the brain tissue but still within the skull, and precisely between the arachnoid mater and pia mater (the delicate innermost layer of the three layers of the meninges that surround the brain).
The above two main types of hemorrhagic stroke are also two different forms of intracranial hemorrhage, which is the accumulation of blood anywhere within the cranial vault; but the other forms of intracranial hemorrhage, such as epidural hematoma (bleeding between the skull and the dura mater, which is the thick outermost layer of the meninges that surround the brain) and subdural hematoma (bleeding in the subdural space), are not considered "hemorrhagic strokes".[30]
Hemorrhagic strokes may occur on the background of alterations to the blood vessels in the brain, such as cerebral amyloid angiopathy, cerebral arteriovenous malformation and an intracranial aneurysm, which can cause intraparenchymal or subarachnoid hemorrhage.[citation needed]
In addition to neurological impairment, hemorrhagic strokes usually cause specific symptoms (for instance, subarachnoid hemorrhage classically causes a severe headache known as a thunderclap headache) or reveal evidence of a previous head injury.
Signs and symptoms
Stroke symptoms typically start suddenly, over seconds to minutes, and in most cases do not progress further. The symptoms depend on the area of the brain affected. The more extensive the area of the brain affected, the more functions that are likely to be lost. Some forms of stroke can cause additional symptoms. For example, in intracranial hemorrhage, the affected area may compress other structures. Most forms of stroke are not associated with a headache, apart from subarachnoid hemorrhage and cerebral venous thrombosis and occasionally intracerebral hemorrhage.[citation needed]
Early recognition
Various systems have been proposed to increase recognition of stroke. Different findings are able to predict the presence or absence of stroke to different degrees. Sudden-onset face weakness, arm drift (i.e., if a person, when asked to raise both arms, involuntarily lets one arm drift downward) and abnormal speech are the findings most likely to lead to the correct identification of a case of stroke, increasing the likelihood by 5.5 when at least one of these is present. Similarly, when all three of these are absent, the likelihood of stroke is decreased (– likelihood ratio of 0.39).[31] While these findings are not perfect for diagnosing stroke, the fact that they can be evaluated relatively rapidly and easily make them very valuable in the acute setting.
A mnemonic to remember the warning signs of stroke is FAST (facial droop, arm weakness, speech difficulty, and time to call emergency services),[32] as advocated by the Department of Health (United Kingdom) and the Stroke Association, the American Stroke Association, the National Stroke Association (US), the Los Angeles Prehospital Stroke Screen (LAPSS)[33] and the Cincinnati Prehospital Stroke Scale (CPSS).[34] Use of these scales is recommended by professional guidelines.[35] FAST is less reliable in the recognition of posterior circulation strokes.[36]
For people referred to the emergency room, early recognition of stroke is deemed important as this can expedite diagnostic tests and treatments. A scoring system called ROSIER (recognition of stroke in the emergency room) is recommended for this purpose; it is based on features from the medical history and physical examination.[35][37]
Subtypes
If the area of the brain affected includes one of the three prominent central nervous system pathways—the spinothalamic tract, corticospinal tract, and the dorsal column–medial lemniscus pathway, symptoms may include:
- hemiplegia and muscle weakness of the face
- numbness
- reduction in sensory or vibratory sensation
- initial flaccidity (reduced muscle tone), replaced by spasticity (increased muscle tone), excessive reflexes, and obligatory synergies.[38]
In most cases, the symptoms affect only one side of the body (unilateral). Depending on the part of the brain affected, the defect in the brain is usually on the opposite side of the body. However, since these pathways also travel in the spinal cord and any lesion there can also produce these symptoms, the presence of any one of these symptoms does not necessarily indicate a stroke. In addition to the above CNS pathways, the brainstem gives rise to most of the twelve cranial nerves. A brainstem stroke affecting the brainstem and brain, therefore, can produce symptoms relating to deficits in these cranial nerves:[citation needed]
- altered smell, taste, hearing, or vision (total or partial)
- drooping of eyelid (ptosis) and weakness of ocular muscles
- decreased reflexes: gag, swallow, pupil reactivity to light
- decreased sensation and muscle weakness of the face
- balance problems and nystagmus
- altered breathing and heart rate
- weakness in sternocleidomastoid muscle with inability to turn head to one side
- weakness in tongue (inability to stick out the tongue or move it from side to side)
If the cerebral cortex is involved, the CNS pathways can again be affected, but also can produce the following symptoms:
- aphasia (difficulty with verbal expression, auditory comprehension, reading and writing; Broca's or Wernicke's area typically involved)
- dysarthria (motor speech disorder resulting from neurological injury)
- apraxia (altered voluntary movements)
- visual field defect
- memory deficits (involvement of temporal lobe)
- hemineglect (involvement of parietal lobe)
- disorganized thinking, confusion, hypersexual gestures (with involvement of frontal lobe)
- lack of insight of his or her, usually stroke-related, disability
If the cerebellum is involved, ataxia might be present and this includes:
- altered walking gait
- altered movement coordination
- vertigo and or disequilibrium
Associated symptoms
Loss of consciousness, headache, and vomiting usually occur more often in hemorrhagic stroke than in thrombosis because of the increased intracranial pressure from the leaking blood compressing the brain.
If symptoms are maximal at onset, the cause is more likely to be a subarachnoid hemorrhage or an embolic stroke.
Causes
Thrombotic stroke
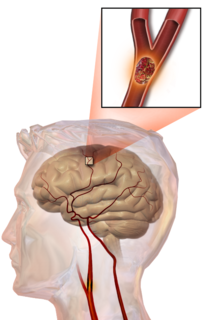
In thrombotic stroke, a thrombus[39] (blood clot) usually forms around atherosclerotic plaques. Since blockage of the artery is gradual, onset of symptomatic thrombotic strokes is slower than that of a hemorrhagic stroke. A thrombus itself (even if it does not completely block the blood vessel) can lead to an embolic stroke (see below) if the thrombus breaks off and travels in the bloodstream, at which point it is called an embolus. Two types of thrombosis can cause stroke:
- Large vessel disease involves the common and internal carotid arteries, the vertebral artery, and the Circle of Willis.[40] Diseases that may form thrombi in the large vessels include (in descending incidence): atherosclerosis, vasoconstriction (tightening of the artery), aortic, carotid or vertebral artery dissection, various inflammatory diseases of the blood vessel wall (Takayasu arteritis, giant cell arteritis, vasculitis), noninflammatory vasculopathy, Moyamoya disease and fibromuscular dysplasia.
- Small vessel disease involves the smaller arteries inside the brain: branches of the circle of Willis, middle cerebral artery, stem, and arteries arising from the distal vertebral and basilar artery.[41] Diseases that may form thrombi in the small vessels include (in descending incidence): lipohyalinosis (build-up of fatty hyaline matter in the blood vessel as a result of high blood pressure and aging) and fibrinoid degeneration (a stroke involving these vessels is known as a lacunar stroke) and microatheroma (small atherosclerotic plaques).[42]
Sickle-cell anemia, which can cause blood cells to clump up and block blood vessels, can also lead to stroke. A stroke is the second leading cause of death in people under 20 with sickle-cell anemia.[43] Air pollution may also increase stroke risk.[44]
Embolic stroke
An embolic stroke refers to an arterial embolism (a blockage of an artery) by an embolus, a traveling particle or debris in the arterial bloodstream originating from elsewhere. An embolus is most frequently a thrombus, but it can also be a number of other substances including fat (e.g., from bone marrow in a broken bone), air, cancer cells or clumps of bacteria (usually from infectious endocarditis).[45]
Because an embolus arises from elsewhere, local therapy solves the problem only temporarily. Thus, the source of the embolus must be identified. Because the embolic blockage is sudden in onset, symptoms usually are maximal at the start. Also, symptoms may be transient as the embolus is partially resorbed and moves to a different location or dissipates altogether.
Emboli most commonly arise from the heart (especially in atrial fibrillation) but may originate from elsewhere in the arterial tree. In paradoxical embolism, a deep vein thrombosis embolizes through an atrial or ventricular septal defect in the heart into the brain.[45]
Causes of stroke related to the heart can be distinguished between high and low-risk:[46]
- High risk: atrial fibrillation and paroxysmal atrial fibrillation, rheumatic disease of the mitral or aortic valve disease, artificial heart valves, known cardiac thrombus of the atrium or ventricle, sick sinus syndrome, sustained atrial flutter, recent myocardial infarction, chronic myocardial infarction together with ejection fraction <28 percent, symptomatic congestive heart failure with ejection fraction <30 percent, dilated cardiomyopathy, Libman-Sacks endocarditis, Marantic endocarditis, infective endocarditis, papillary fibroelastoma, left atrial myxoma and coronary artery bypass graft (CABG) surgery.
- Low risk/potential: calcification of the annulus (ring) of the mitral valve, patent foramen ovale (PFO), atrial septal aneurysm, atrial septal aneurysm with patent foramen ovale, left ventricular aneurysm without thrombus, isolated left atrial "smoke" on echocardiography (no mitral stenosis or atrial fibrillation), complex atheroma in the ascending aorta or proximal arch.
Among those who have a complete blockage of one of the carotid arteries, the risk of stroke on that side is about one percent per year.[47]
A special form of embolic stroke is the embolic stroke of undetermined source (ESUS). This subset of cryptogenetic stroke is defined as a non-lacunar brain infarct without proximal arterial stenosis or cardioembolic sources. About one out of six ischemic strokes could be classified as ESUS.[48]
Cerebral hypoperfusion
Cerebral hypoperfusion is the reduction of blood flow to all parts of the brain. The reduction could be to a particular part of the brain depending on the cause. It is most commonly due to heart failure from cardiac arrest or arrhythmias, or from reduced cardiac output as a result of myocardial infarction, pulmonary embolism, pericardial effusion, or bleeding.[citation needed] Hypoxemia (low blood oxygen content) may precipitate the hypoperfusion. Because the reduction in blood flow is global, all parts of the brain may be affected, especially vulnerable "watershed" areas—border zone regions supplied by the major cerebral arteries. A watershed stroke refers to the condition when the blood supply to these areas is compromised. Blood flow to these areas does not necessarily stop, but instead it may lessen to the point where brain damage can occur.
Venous thrombosis
Cerebral venous sinus thrombosis leads to stroke due to locally increased venous pressure, which exceeds the pressure generated by the arteries. Infarcts are more likely to undergo hemorrhagic transformation (leaking of blood into the damaged area) than other types of ischemic stroke.[22]
Intracerebral bleed
It generally occurs in small arteries or arterioles and is commonly due to hypertension,[49] intracranial vascular malformations (including cavernous angiomas or arteriovenous malformations), cerebral amyloid angiopathy, or infarcts into which secondary hemorrhage has occurred.[2] Other potential causes are trauma, bleeding disorders, amyloid angiopathy, illicit drug use (e.g., amphetamines or cocaine). The hematoma enlarges until pressure from surrounding tissue limits its growth, or until it decompresses by emptying into the ventricular system, CSF or the pial surface. A third of intracerebral bleed is into the brain's ventricles. ICH has a mortality rate of 44 percent after 30 days, higher than ischemic stroke or subarachnoid hemorrhage (which technically may also be classified as a type of stroke[2]).
Other
Other causes may include spasm of an artery. This may occur due to cocaine.[50]
Silent stroke
A silent stroke is a stroke that does not have any outward symptoms, and people are typically unaware they have had a stroke. Despite not causing identifiable symptoms, a silent stroke still damages the brain and places the person at increased risk for both transient ischemic attack and major stroke in the future. Conversely, those who have had a major stroke are also at risk of having silent strokes.[51] In a broad study in 1998, more than 11 million people were estimated to have experienced a stroke in the United States. Approximately 770,000 of these strokes were symptomatic and 11 million were first-ever silent MRI infarcts or hemorrhages. Silent strokes typically cause lesions which are detected via the use of neuroimaging such as MRI. Silent strokes are estimated to occur at five times the rate of symptomatic strokes.[52][53] The risk of silent stroke increases with age, but may also affect younger adults and children, especially those with acute anemia.[52][54]
Pathophysiology
Ischemic
Ischemic stroke occurs because of a loss of blood supply to part of the brain, initiating the ischemic cascade.[56] Brain tissue ceases to function if deprived of oxygen for more than 60 to 90 seconds[citation needed], and after approximately three hours will suffer irreversible injury possibly leading to the death of the tissue, i.e., infarction. (This is why fibrinolytics such as alteplase are given only until three hours since the onset of the stroke.) Atherosclerosis may disrupt the blood supply by narrowing the lumen of blood vessels leading to a reduction of blood flow, by causing the formation of blood clots within the vessel, or by releasing showers of small emboli through the disintegration of atherosclerotic plaques.[57] Embolic infarction occurs when emboli formed elsewhere in the circulatory system, typically in the heart as a consequence of atrial fibrillation, or in the carotid arteries, break off, enter the cerebral circulation, then lodge in and block brain blood vessels. Since blood vessels in the brain are now blocked, the brain becomes low in energy, and thus it resorts to using anaerobic metabolism within the region of brain tissue affected by ischemia. Anaerobic metabolism produces less adenosine triphosphate (ATP) but releases a by-product called lactic acid. Lactic acid is an irritant which could potentially destroy cells since it is an acid and disrupts the normal acid-base balance in the brain. The ischemia area is referred to as the "ischemic penumbra".[58]
As oxygen or glucose becomes depleted in ischemic brain tissue, the production of high energy phosphate compounds such as adenosine triphosphate (ATP) fails, leading to failure of energy-dependent processes (such as ion pumping) necessary for tissue cell survival. This sets off a series of interrelated events that result in cellular injury and death. A major cause of neuronal injury is the release of the excitatory neurotransmitter glutamate. The concentration of glutamate outside the cells of the nervous system is normally kept low by so-called uptake carriers, which are powered by the concentration gradients of ions (mainly Na+) across the cell membrane. However, stroke cuts off the supply of oxygen and glucose which powers the ion pumps maintaining these gradients. As a result, the transmembrane ion gradients run down, and glutamate transporters reverse their direction, releasing glutamate into the extracellular space. Glutamate acts on receptors in nerve cells (especially NMDA receptors), producing an influx of calcium which activates enzymes that digest the cells' proteins, lipids, and nuclear material. Calcium influx can also lead to the failure of mitochondria, which can lead further toward energy depletion and may trigger cell death due to programmed cell death.[59]
Ischemia also induces production of oxygen free radicals and other reactive oxygen species. These react with and damage a number of cellular and extracellular elements. Damage to the blood vessel lining or endothelium is particularly important. In fact, many antioxidant neuroprotectants such as uric acid and NXY-059 work at the level of the endothelium and not in the brain per se. Free radicals also directly initiate elements of the programmed cell death cascade by means of redox signaling.[60]
These processes are the same for any type of ischemic tissue and are referred to collectively as the ischemic cascade. However, brain tissue is especially vulnerable to ischemia since it has little respiratory reserve and is completely dependent on aerobic metabolism, unlike most other organs.
In addition to damaging effects on brain cells, ischemia and infarction can result in loss of structural integrity of brain tissue and blood vessels, partly through the release of matrix metalloproteases, which are zinc- and calcium-dependent enzymes that break down collagen, hyaluronic acid, and other elements of connective tissue. Other proteases also contribute to this process. The loss of vascular structural integrity results in a breakdown of the protective blood brain barrier that contributes to cerebral edema, which can cause secondary progression of the brain injury.[citation needed]
Hemorrhagic
Hemorrhagic strokes are classified based on their underlying pathology. Some causes of hemorrhagic stroke are hypertensive hemorrhage, ruptured aneurysm, ruptured AV fistula, transformation of prior ischemic infarction, and drug-induced bleeding.[61] They result in tissue injury by causing compression of tissue from an expanding hematoma or hematomas. In addition, the pressure may lead to a loss of blood supply to affected tissue with resulting infarction, and the blood released by brain hemorrhage appears to have direct toxic effects on brain tissue and vasculature.[43][62] Inflammation contributes to the secondary brain injury after hemorrhage.[62]
Diagnosis


Stroke is diagnosed through several techniques: a neurological examination (such as the NIHSS), CT scans (most often without contrast enhancements) or MRI scans, Doppler ultrasound, and arteriography. The diagnosis of stroke itself is clinical, with assistance from the imaging techniques. Imaging techniques also assist in determining the subtypes and cause of stroke. There is yet no commonly used blood test for the stroke diagnosis itself, though blood tests may be of help in finding out the likely cause of stroke.[63]
Physical examination
A physical examination, including taking a medical history of the symptoms and a neurological status, helps giving an evaluation of the location and severity of a stroke. It can give a standard score on e.g., the NIH stroke scale.
Imaging
For diagnosing ischemic (blockage) stroke in the emergency setting:[64]
- CT scans (without contrast enhancements)
- sensitivity= 16% (less than 10% within first 3 hours of symptom onset)
- specificity= 96%
- MRI scan
- sensitivity= 83%
- specificity= 98%
For diagnosing hemorrhagic stroke in the emergency setting:
- CT scans (without contrast enhancements)
- sensitivity= 89%
- specificity= 100%
- MRI scan
- sensitivity= 81%
- specificity= 100%
For detecting chronic hemorrhages, MRI scan is more sensitive.[65]
For the assessment of stable stroke, nuclear medicine scans SPECT and PET/CT may be helpful. SPECT documents cerebral blood flow and PET with FDG isotope the metabolic activity of the neurons.
CT scans may not detect an ischemic stroke, especially if it is small, of recent onset, or in the brainstem or cerebellum areas. A CT scan is more to rule out certain stroke mimics and detect bleeding.[9]
Underlying cause

When a stroke has been diagnosed, various other studies may be performed to determine the underlying cause. With the current treatment and diagnosis options available, it is of particular importance to determine whether there is a peripheral source of emboli. Test selection may vary since the cause of stroke varies with age, comorbidity and the clinical presentation. The following are commonly used techniques:
- an ultrasound/doppler study of the carotid arteries (to detect carotid stenosis) or dissection of the precerebral arteries;
- an electrocardiogram (ECG) and echocardiogram (to identify arrhythmias and resultant clots in the heart which may spread to the brain vessels through the bloodstream);
- a Holter monitor study to identify intermittent abnormal heart rhythms;
- an angiogram of the cerebral vasculature (if a bleed is thought to have originated from an aneurysm or arteriovenous malformation);
- blood tests to determine if blood cholesterol is high, if there is an abnormal tendency to bleed, and if some rarer processes such as homocystinuria might be involved.
For hemorrhagic strokes, a CT or MRI scan with intravascular contrast may be able to identify abnormalities in the brain arteries (such as aneurysms) or other sources of bleeding, and structural MRI if this shows no cause. If this too does not identify an underlying reason for the bleeding, invasive cerebral angiography could be performed but this requires access to the bloodstream with an intravascular catheter and can cause further strokes as well as complications at the insertion site and this investigation is therefore reserved for specific situations.[66] If there are symptoms suggesting that the hemorrhage might have occurred as a result of venous thrombosis, CT or MRI venography can be used to examine the cerebral veins.[66]
Misdiagnosis
Among people with ischemic strokes, misdiagnosis occurs 2 to 26% of the time.[67] A "stroke chameleon" (SC) is stroke which is diagnosed as something else.[67][68]
People not having a stroke may also be misdiagnosed as a stroke. Giving thrombolytics (clot-busting) in such cases causes intracerebral bleeding 1 to 2% of the time, which is less than that of people with strokes. This unnecessary treatment adds to health care costs. Even so, the AHA/ASA guidelines state that starting intravenous tPA in possible mimics is preferred to delaying treatment for additional testing.[67]
Women, African-Americans, Hispanic-Americans, Asian and Pacific Islanders are more often misdiagnosed for a condition other than stroke when in fact having a stroke. In addition, adults under 44 years-of-age are seven times more likely to have a stroke missed than are adults over 75 years-of-age. This is especially the case for younger people with posterior circulation infarcts.[67] Some medical centers have used hyperacute MRI in experimental studies for persons initially thought to have a low likelihood of stroke. And in some of these persons, strokes have been found which were then treated with thrombolytic medication.[67]
Prevention
Given the disease burden of strokes, prevention is an important public health concern.[69] Primary prevention is less effective than secondary prevention (as judged by the number needed to treat to prevent one stroke per year).[69] Recent guidelines detail the evidence for primary prevention in stroke.[70] In those who are otherwise healthy, aspirin does not appear beneficial and thus is not recommended.[71] In people who have had a myocardial infarction or those with a high cardiovascular risk, it provides some protection against a first stroke.[72][73] In those who have previously had a stroke, treatment with medications such as aspirin, clopidogrel, and dipyridamole may be beneficial.[72] The U.S. Preventive Services Task Force (USPSTF) recommends against screening for carotid artery stenosis in those without symptoms.[74]
Risk factors
The most important modifiable risk factors for stroke are high blood pressure and atrial fibrillation although the size of the effect is small with 833 people have to be treated for 1 year to prevent one stroke.[75][76] Other modifiable risk factors include high blood cholesterol levels, diabetes mellitus, end-stage kidney disease,[7] cigarette smoking[77][78] (active and passive), heavy alcohol use,[79] drug use,[80] lack of physical activity, obesity, processed red meat consumption,[81] and unhealthy diet.[82] Smoking just one cigarette per day increases the risk more than 30%.[83] Alcohol use could predispose to ischemic stroke, as well as intracerebral and subarachnoid hemorrhage via multiple mechanisms (for example, via hypertension, atrial fibrillation, rebound thrombocytosis and platelet aggregation and clotting disturbances).[84] Drugs, most commonly amphetamines and cocaine, can induce stroke through damage to the blood vessels in the brain and acute hypertension.[85][86] Migraine with aura doubles a person's risk for ischemic stroke.[87][88] Untreated, celiac disease regardless of the presence of symptoms can be an underlying cause of stroke, both in children and adults.[89]
High levels of physical activity reduce the risk of stroke by about 26%.[90] There is a lack of high quality studies looking at promotional efforts to improve lifestyle factors.[91] Nonetheless, given the large body of circumstantial evidence, best medical management for stroke includes advice on diet, exercise, smoking and alcohol use.[92] Medication is the most common method of stroke prevention; carotid endarterectomy can be a useful surgical method of preventing stroke.
Blood pressure
High blood pressure accounts for 35–50% of stroke risk.[93] Blood pressure reduction of 10 mmHg systolic or 5 mmHg diastolic reduces the risk of stroke by ~40%.[94] Lowering blood pressure has been conclusively shown to prevent both ischemic and hemorrhagic strokes.[95][96] It is equally important in secondary prevention.[97] Even people older than 80 years and those with isolated systolic hypertension benefit from antihypertensive therapy.[98][99][100] The available evidence does not show large differences in stroke prevention between antihypertensive drugs—therefore, other factors such as protection against other forms of cardiovascular disease and cost should be considered.[101][102] The routine use of beta-blockers following a stroke or TIA has not been shown to result in benefits.[103]
Blood lipids
High cholesterol levels have been inconsistently associated with (ischemic) stroke.[96][104] Statins have been shown to reduce the risk of stroke by about 15%.[105] Since earlier meta-analyses of other lipid-lowering drugs did not show a decreased risk,[106] statins might exert their effect through mechanisms other than their lipid-lowering effects.[105]
Diabetes mellitus
Diabetes mellitus increases the risk of stroke by 2 to 3 times. While intensive blood sugar control has been shown to reduce small blood vessel complications such as kidney damage and damage to the retina of the eye it has not been shown to reduce large blood vessel complications such as stroke.[107][108]
Anticoagulation drugs
Oral anticoagulants such as warfarin have been the mainstay of stroke prevention for over 50 years. However, several studies have shown that aspirin and other antiplatelets are highly effective in secondary prevention after a stroke or transient ischemic attack.[72] Low doses of aspirin (for example 75–150 mg) are as effective as high doses but have fewer side effects; the lowest effective dose remains unknown.[109] Thienopyridines (clopidogrel, ticlopidine) might be slightly more effective than aspirin and have a decreased risk of gastrointestinal bleeding, but are more expensive.[110] Both aspirin and clopidogrel may be useful in the first few weeks after a minor stroke or high risk TIA.[111] Clopidogrel has less side effects than ticlopidine.[110] Dipyridamole can be added to aspirin therapy to provide a small additional benefit, even though headache is a common side effect.[112] Low-dose aspirin is also effective for stroke prevention after having a myocardial infarction.[73]
Those with atrial fibrillation have a 5% a year risk of stroke, and this risk is higher in those with valvular atrial fibrillation.[113] Depending on the stroke risk, anticoagulation with medications such as warfarin or aspirin is useful for prevention.[114] Except in people with atrial fibrillation, oral anticoagulants are not advised for stroke prevention—any benefit is offset by bleeding risk.[115]
In primary prevention, however, antiplatelet drugs did not reduce the risk of ischemic stroke but increased the risk of major bleeding.[116][117] Further studies are needed to investigate a possible protective effect of aspirin against ischemic stroke in women.[118][119]
Surgery
Carotid endarterectomy or carotid angioplasty can be used to remove atherosclerotic narrowing of the carotid artery. There is evidence supporting this procedure in selected cases.[92] Endarterectomy for a significant stenosis has been shown to be useful in preventing further strokes in those who have already had one.[120] Carotid artery stenting has not been shown to be equally useful.[121][122] People are selected for surgery based on age, gender, degree of stenosis, time since symptoms and the person's preferences.[92] Surgery is most efficient when not delayed too long—the risk of recurrent stroke in a person who has a 50% or greater stenosis is up to 20% after 5 years, but endarterectomy reduces this risk to around 5%. The number of procedures needed to cure one person was 5 for early surgery (within two weeks after the initial stroke), but 125 if delayed longer than 12 weeks.[123][124]
Screening for carotid artery narrowing has not been shown to be a useful test in the general population.[125] Studies of surgical intervention for carotid artery stenosis without symptoms have shown only a small decrease in the risk of stroke.[126][127] To be beneficial, the complication rate of the surgery should be kept below 4%. Even then, for 100 surgeries, 5 people will benefit by avoiding stroke, 3 will develop stroke despite surgery, 3 will develop stroke or die due to the surgery itself, and 89 will remain stroke-free but would also have done so without intervention.[92]
Diet
Nutrition, specifically the Mediterranean-style diet, has the potential for decreasing the risk of having a stroke by more than half.[128] It does not appear that lowering levels of homocysteine with folic acid affects the risk of stroke.[129][130]
Women
A number of specific recommendations have been made for women including taking aspirin after the 11th week of pregnancy if there is a history of previous chronic high blood pressure and taking blood pressure medications during pregnancy if the blood pressure is greater than 150 mmHg systolic or greater than 100 mmHg diastolic. In those who have previously had preeclampsia other risk factors should be treated more aggressively.[131]
Previous stroke or TIA
Keeping blood pressure below 140/90 mmHg is recommended.[132] Anticoagulation can prevent recurrent ischemic strokes. Among people with nonvalvular atrial fibrillation, anticoagulation can reduce stroke by 60% while antiplatelet agents can reduce stroke by 20%.[133] However, a recent meta-analysis suggests harm from anticoagulation started early after an embolic stroke.[134] Stroke prevention treatment for atrial fibrillation is determined according to the CHA2DS2–VASc score. The most widely used anticoagulant to prevent thromboembolic stroke in people with nonvalvular atrial fibrillation is the oral agent warfarin while a number of newer agents including dabigatran are alternatives which do not require prothrombin time monitoring.[132]
Anticoagulants, when used following stroke, should not be stopped for dental procedures.[135]
If studies show carotid artery stenosis, and the person has a degree of residual function on the affected side, carotid endarterectomy (surgical removal of the stenosis) may decrease the risk of recurrence if performed rapidly after stroke.
Management
Ischemic stroke
Aspirin reduces the overall risk of recurrence by 13% with greater benefit early on.[136] Definitive therapy within the first few hours is aimed at removing the blockage by breaking the clot down (thrombolysis), or by removing it mechanically (thrombectomy). The premise underlying the importance of rapid stroke intervention was summed up as Time is Brain! in the early 1990s.[137] Years later, that same idea, that rapid cerebral blood flow restoration results in fewer brain cells dying, has been proved and quantified.[138] Dual antiplatelet therapy, with aspirin and clopidogrel for 30 days, decreases the risk of a subsequent stroke more than it increases bleeding with aspirin alone.[139][140]
Tight blood sugar control in the first few hours does not improve outcomes and may cause harm.[141] High blood pressure is also not typically lowered as this has not been found to be helpful.[142][143] Cerebrolysin, a mix of pig brain tissue used to treat acute ischemic stroke in many Asian and European countries, does not improve outcomes and may increase the risk of severe side effects.[144]
Thrombolysis
Thrombolysis, such as with recombinant tissue plasminogen activator (rtPA), in acute ischemic stroke, when given within three hours of symptom onset, results in an overall benefit of 10% with respect to living without disability.[145][146] It does not, however, improve chances of survival.[145] Benefit is greater the earlier it is used.[145] Between three and four and a half hours the effects are less clear.[147][148][149] The AHA/ASA recommend it for certain people in this time frame.[150] A 2014 review found a 5% increase in the number of people living without disability at three to six months; however, there was a 2% increased risk of death in the short term.[146] After four and a half hours thrombolysis worsens outcomes.[147] These benefits or lack of benefits occurred regardless of the age of the person treated.[151] There is no reliable way to determine who will have an intracranial bleed post-treatment versus who will not.[152] In those with findings of savable tissue on medical imaging between 4.5 hours and 9 hours or who wake up with a stroke, alteplase results in some benefit.[153] In those with a wake up stroke; however, it also results in more deaths than placebo.[154]
Its use is endorsed by the American Heart Association, the American College of Emergency Physicians and the American Academy of Neurology as the recommended treatment for acute stroke within three hours of onset of symptoms as long as there are no other contraindications (such as abnormal lab values, high blood pressure, or recent surgery). This position for tPA is based upon the findings of two studies by one group of investigators[155] which showed that tPA improves the chances for a good neurological outcome. When administered within the first three hours thrombolysis improves functional outcome without affecting mortality.[156] 6.4% of people with large strokes developed substantial brain bleeding as a complication from being given tPA thus part of the reason for increased short term mortality.[157] The American Academy of Emergency Medicine had previously stated that objective evidence regarding the applicability of tPA for acute ischemic stroke was insufficient.[158] In 2013 the American College of Emergency Medicine refuted this position,[159] acknowledging the body of evidence for the use of tPA in ischemic stroke;[160] but debate continues.[161][162] Intra-arterial fibrinolysis, where a catheter is passed up an artery into the brain and the medication is injected at the site of thrombosis, has been found to improve outcomes in people with acute ischemic stroke.[163]
Endovascular treatment
Mechanical removal of the blood clot causing the ischemic stroke, called mechanical thrombectomy, is a potential treatment for occlusion of a large artery, such as the middle cerebral artery. In 2015, one review demonstrated the safety and efficacy of this procedure if performed within 12 hours of the onset of symptoms.[164][165] It did not change the risk of death, but reduced disability compared to the use of intravenous thrombolysis which is generally used in people evaluated for mechanical thrombectomy.[166][167] Certain cases may benefit from thrombectomy up to 24 hours after the onset of symptoms.[168]
Craniectomy
Strokes affecting large portions of the brain can cause significant brain swelling with secondary brain injury in surrounding tissue. This phenomenon is mainly encountered in strokes affecting brain tissue dependent upon the middle cerebral artery for blood supply and is also called "malignant cerebral infarction" because it carries a dismal prognosis. Relief of the pressure may be attempted with medication, but some require hemicraniectomy, the temporary surgical removal of the skull on one side of the head. This decreases the risk of death, although some people – who would otherwise have died – survive with disability.[169]
Bleeding stroke
People with intracerebral hemorrhage require supportive care, including blood pressure control if required. People are monitored for changes in the level of consciousness, and their blood sugar and oxygenation are kept at optimum levels. Anticoagulants and antithrombotics can make bleeding worse and are generally discontinued (and reversed if possible).[citation needed] A proportion may benefit from neurosurgical intervention to remove the blood and treat the underlying cause, but this depends on the location and the size of the hemorrhage as well as patient-related factors, and ongoing research is being conducted into the question as to which people with intracerebral hemorrhage may benefit.[170]
In subarachnoid hemorrhage, early treatment for underlying cerebral aneurysms may reduce the risk of further hemorrhages. Depending on the site of the aneurysm this may be by surgery that involves opening the skull or endovascularly (through the blood vessels).[171]
Stroke unit
Ideally, people who have had a stroke are admitted to a "stroke unit", a dedicated area in a hospital staffed by nurses and therapists with experience in stroke treatment. People admitted to a stroke unit have a higher chance of surviving than those admitted elsewhere in hospital, even if they are being cared for by doctors without experience in stroke.[2][172] Nursing care is fundamental in maintaining skin care, feeding, hydration, positioning, and monitoring vital signs such as temperature, pulse, and blood pressure.[173]
Rehabilitation
Stroke rehabilitation is the process by which those with disabling strokes undergo treatment to help them return to normal life as much as possible by regaining and relearning the skills of everyday living. It also aims to help the survivor understand and adapt to difficulties, prevent secondary complications, and educate family members to play a supporting role. Stroke rehabilitation should begin almost immediately with a multidisciplinary approach. The rehabilitation team may involve physicians trained in rehabilitation medicine, neurologists, clinical pharmacists, nursing staff, physiotherapists, occupational therapists, speech-language pathologists, and orthotists. Some teams may also include psychologists and social workers, since at least one-third of affected people manifests post stroke depression. Validated instruments such as the Barthel scale may be used to assess the likelihood of a person who has had a stroke being able to manage at home with or without support subsequent to discharge from a hospital.[174]
Stroke rehabilitation should be started as quickly as possible and can last anywhere from a few days to over a year. Most return of function is seen in the first few months, and then improvement falls off with the "window" considered officially by U.S. state rehabilitation units and others to be closed after six months, with little chance of further improvement.[medical citation needed] However, some people have reported that they continue to improve for years, regaining and strengthening abilities like writing, walking, running, and talking.[medical citation needed] Daily rehabilitation exercises should continue to be part of the daily routine for people who have had a stroke. Complete recovery is unusual but not impossible and most people will improve to some extent: proper diet and exercise are known to help the brain to recover.
Physical therapy
Physical and occupational therapy have overlapping areas of expertise; however, physical therapy focuses on joint range of motion and strength by performing exercises and relearning functional tasks such as bed mobility, transferring, walking and other gross motor functions. Physiotherapists can also work with people who have had a stroke to improve awareness and use of the hemiplegic side. Rehabilitation involves working on the ability to produce strong movements or the ability to perform tasks using normal patterns. Emphasis is often concentrated on functional tasks and people's goals. One example physiotherapists employ to promote motor learning involves constraint-induced movement therapy. Through continuous practice the person relearns to use and adapt the hemiplegic limb during functional activities to create lasting permanent changes.[175] Physical therapy is effective for recovery of function and mobility after stroke.[176] Occupational therapy is involved in training to help relearn everyday activities known as the activities of daily living (ADLs) such as eating, drinking, dressing, bathing, cooking, reading and writing, and toileting. Approaches to helping people with urinary incontinence include physical therapy, cognitive therapy, and specialized interventions with experienced medical professionals, however, it is not clear how effective these approaches are at improving urinary incontinence following a stroke.[177]
Treatment of spasticity related to stroke often involves early mobilizations, commonly performed by a physiotherapist, combined with elongation of spastic muscles and sustained stretching through various different positions.[38] Gaining initial improvement in range of motion is often achieved through rhythmic rotational patterns associated with the affected limb.[38] After full range has been achieved by the therapist, the limb should be positioned in the lengthened positions to prevent against further contractures, skin breakdown, and disuse of the limb with the use of splints or other tools to stabilize the joint.[38] Cold in the form of ice wraps or ice packs have been proven to briefly reduce spasticity by temporarily dampening neural firing rates.[38] Electrical stimulation to the antagonist muscles or vibrations has also been used with some success.[38] Physical therapy is sometimes suggested for people who experience sexual dysfunction following a stroke.[178]
Speech therapy
Speech and language therapy is appropriate for people with the speech production disorders: dysarthria[179] and apraxia of speech,[180] aphasia,[181] cognitive-communication impairments, and problems with swallowing. Speech and language therapy for aphasia following stroke compared to no therapy improves functional communication, reading, writing and expressive language. There may be benefit in high intensity and high doses over a longer period, but these higher intensity doses may not be acceptable to everyone.[176]
People who have had a stroke may have particular problems, such as dysphagia, which can cause swallowed material to pass into the lungs and cause aspiration pneumonia. The condition may improve with time, but in the interim, a nasogastric tube may be inserted, enabling liquid food to be given directly into the stomach. If swallowing is still deemed unsafe, then a percutaneous endoscopic gastrostomy (PEG) tube is passed and this can remain indefinitely. Swallowing therapy has mixed results as of 2018.[182]
Devices
Often, assistive technology such as wheelchairs, walkers and canes may be beneficial. Many mobility problems can be improved by the use of ankle foot orthoses.[183]
Physical fitness
A stroke can also reduce people's general fitness.[184] Reduced fitness can reduce capacity for rehabilitation as well as general health.[185] Physical exercises as part of a rehabilitation program following a stroke appear safe.[184] Cardiorespiratory fitness training that involves walking in rehabilitation can improve speed, tolerance and independence during walking, and may improve balance.[184] There are inadequate long-term data about the effects of exercise and training on death, dependence and disability after a stroke.[184] The future areas of research may concentrate on the optimal exercise prescription and long-term health benefits of exercise. The effect of physical training on cognition also may be studied further.
The ability to walk independently in their community, indoors or outdoors, is important following stroke. Although no negative effects have been reported, it is unclear if outcomes can improve with these walking programs when compared to usual treatment.[186]
Other therapy methods
Some current and future therapy methods include the use of virtual reality and video games for rehabilitation. These forms of rehabilitation offer potential for motivating people to perform specific therapy tasks that many other forms do not.[187] While virtual reality and interactive video gaming are not more effective than conventional therapy for improving upper limb function, when used in conjunction with usual care these approaches may improve upper limb function and ADL function.[188] There are inadequate data on the effect of virtual reality and interactive video gaming on gait speed, balance, participation and quality of life.[188] Many clinics and hospitals are adopting the use of these off-the-shelf devices for exercise, social interaction, and rehabilitation because they are affordable, accessible and can be used within the clinic and home.[187]
Mirror therapy is associated with improved motor function of the upper extremity in people who have had a stroke.[189]
Other non-invasive rehabilitation methods used to augment physical therapy of motor function in people recovering from a stroke include transcranial magnetic stimulation and transcranial direct-current stimulation.[190] and robotic therapies.[191] Constraint‐induced movement therapy (CIMT), mental practice, mirror therapy, interventions for sensory impairment, virtual reality and a relatively high dose of repetitive task practice may be effective in improving upper limb function. However, further primary research, specifically of CIMT, mental practice, mirror therapy and virtual reality is needed.[192]
Self-management
A stroke can affect the ability to live independently and with quality. Self-management programs are a special training that educates stroke survivors about stroke and its consequences, helps them acquire skills to cope with their challenges, and helps them set and meet their own goals during their recovery process. These programs are tailored to the target audience, and led by someone trained and expert in stroke and its consequences (most commonly professionals, but also stroke survivors and peers). A 2016 review reported that these programs improve the quality of life after stroke, without negative effects. People with stroke felt more empowered, happy and satisfied with life after participating in this training.[193]
Prognosis
Disability affects 75% of stroke survivors enough to decrease their ability to work.[194] Stroke can affect people physically, mentally, emotionally, or a combination of the three. The results of stroke vary widely depending on size and location of the lesion.[195]
Physical effects
Some of the physical disabilities that can result from stroke include muscle weakness, numbness, pressure sores, pneumonia, incontinence, apraxia (inability to perform learned movements), difficulties carrying out daily activities, appetite loss, speech loss, vision loss and pain. If the stroke is severe enough, or in a certain location such as parts of the brainstem, coma or death can result. Up to 10% of people following a stroke develop seizures, most commonly in the week subsequent to the event; the severity of the stroke increases the likelihood of a seizure.[196][197] An estimated 15% of people experience urinary incontinence for more than a year following a stroke.[177] 50% of people have a decline in sexual function (sexual dysfunction) following a stroke.[178]
Emotional and mental effects
Emotional and mental dysfunctions correspond to areas in the brain that have been damaged. Emotional problems following a stroke can be due to direct damage to emotional centers in the brain or from frustration and difficulty adapting to new limitations. Post-stroke emotional difficulties include anxiety, panic attacks, flat affect (failure to express emotions), mania, apathy and psychosis. Other difficulties may include a decreased ability to communicate emotions through facial expression, body language and voice.[198]
Disruption in self-identity, relationships with others, and emotional well-being can lead to social consequences after stroke due to the lack of ability to communicate. Many people who experience communication impairments after a stroke find it more difficult to cope with the social issues rather than physical impairments. Broader aspects of care must address the emotional impact speech impairment has on those who experience difficulties with speech after a stroke.[199] Those who experience a stroke are at risk of paralysis which could result in a self disturbed body image which may also lead to other social issues.[200]
30 to 50% of stroke survivors suffer post-stroke depression, which is characterized by lethargy, irritability, sleep disturbances, lowered self-esteem and withdrawal.[201] Depression can reduce motivation and worsen outcome, but can be treated with social and family support, psychotherapy and, in severe cases, antidepressants. Psychotherapy sessions may have a small effect on improving mood and preventing depression after a stroke,[202] however psychotherapy does not appear to be effective at treating depression after a stroke.[203] Antidepressant medications may be useful for treating depression after a stroke.[203]
Emotional lability, another consequence of stroke, causes the person to switch quickly between emotional highs and lows and to express emotions inappropriately, for instance with an excess of laughing or crying with little or no provocation. While these expressions of emotion usually correspond to the person's actual emotions, a more severe form of emotional lability causes the affected person to laugh and cry pathologically, without regard to context or emotion.[194] Some people show the opposite of what they feel, for example crying when they are happy.[204] Emotional lability occurs in about 20% of those who have had a stroke. Those with a right hemisphere stroke are more likely to have an empathy problems which can make communication harder.[205]
Cognitive deficits resulting from stroke include perceptual disorders, aphasia,[206] dementia,[207][208] and problems with attention[209] and memory.[210] A stroke sufferer may be unaware of his or her own disabilities, a condition called anosognosia. In a condition called hemispatial neglect, the affected person is unable to attend to anything on the side of space opposite to the damaged hemisphere.Cognitive and psychological outcome after a stroke can be affected by the age at which the stroke happened, pre-stroke baseline intellectual functioning, psychiatric history and whether there is pre-existing brain pathology.[211]
Epidemiology

| no data <250 250–425 425–600 600–775 775–950 950–1125 | 1125–1300 1300–1475 1475–1650 1650–1825 1825–2000 >2000 |
Stroke was the second most frequent cause of death worldwide in 2011, accounting for 6.2 million deaths (~11% of the total).[213] Approximately 17 million people had a stroke in 2010 and 33 million people have previously had a stroke and were still alive.[17] Between 1990 and 2010 the number of strokes decreased by approximately 10% in the developed world and increased by 10% in the developing world.[17] Overall, two-thirds of strokes occurred in those over 65 years old.[17] South Asians are at particularly high risk of stroke, accounting for 40% of global stroke deaths.[214]
It is ranked after heart disease and before cancer.[2] In the United States stroke is a leading cause of disability, and recently declined from the third leading to the fourth leading cause of death.[215] Geographic disparities in stroke incidence have been observed, including the existence of a "stroke belt" in the southeastern United States, but causes of these disparities have not been explained.
The risk of stroke increases exponentially from 30 years of age, and the cause varies by age.[216] Advanced age is one of the most significant stroke risk factors. 95% of strokes occur in people age 45 and older, and two-thirds of strokes occur in those over the age of 65.[43][201] A person's risk of dying if he or she does have a stroke also increases with age. However, stroke can occur at any age, including in childhood.
Family members may have a genetic tendency for stroke or share a lifestyle that contributes to stroke. Higher levels of Von Willebrand factor are more common amongst people who have had ischemic stroke for the first time.[217] The results of this study found that the only significant genetic factor was the person's blood type. Having had a stroke in the past greatly increases one's risk of future strokes.
Men are 25% more likely to suffer strokes than women,[43] yet 60% of deaths from stroke occur in women.[204] Since women live longer, they are older on average when they have their strokes and thus more often killed.[43] Some risk factors for stroke apply only to women. Primary among these are pregnancy, childbirth, menopause, and the treatment thereof (HRT).
History

Episodes of stroke and familial stroke have been reported from the 2nd millennium BC onward in ancient Mesopotamia and Persia.[218] Hippocrates (460 to 370 BC) was first to describe the phenomenon of sudden paralysis that is often associated with ischemia. Apoplexy, from the Greek word meaning "struck down with violence", first appeared in Hippocratic writings to describe this phenomenon.[219][220] The word stroke was used as a synonym for apoplectic seizure as early as 1599,[221] and is a fairly literal translation of the Greek term.
In 1658, in his Apoplexia, Johann Jacob Wepfer (1620–1695) identified the cause of hemorrhagic stroke when he suggested that people who had died of apoplexy had bleeding in their brains.[43][219] Wepfer also identified the main arteries supplying the brain, the vertebral and carotid arteries, and identified the cause of a type of ischemic stroke known as a cerebral infarction when he suggested that apoplexy might be caused by a blockage to those vessels.[43] Rudolf Virchow first described the mechanism of thromboembolism as a major factor.[222]
The term cerebrovascular accident was introduced in 1927, reflecting a "growing awareness and acceptance of vascular theories and (...) recognition of the consequences of a sudden disruption in the vascular supply of the brain".[223] Its use is now discouraged by a number of neurology textbooks, reasoning that the connotation of fortuitousness carried by the word accident insufficiently highlights the modifiability of the underlying risk factors.[224][225][226] Cerebrovascular insult may be used interchangeably.[227]
The term brain attack was introduced for use to underline the acute nature of stroke according to the American Stroke Association,[227] which has used the term since 1990,[228] and is used colloquially to refer to both ischemic as well as hemorrhagic stroke.[229]
Research
As of 2017, angioplasty and stents were under preliminary clinical research to determine the possible therapeutic advantages of these procedures in comparison to therapy with statins, antithrombotics, or antihypertensive drugs.[230]
See also
References
- ↑ Gaillard, Frank. "Ischaemic stroke". radiopaedia.org. Archived from the original on 28 November 2011. Retrieved 3 June 2018.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 Donnan GA, Fisher M, Macleod M, Davis SM (May 2008). "Stroke". The Lancet. 371 (9624): 1612–23. doi:10.1016/S0140-6736(08)60694-7. PMID 18468545.(subscription required)
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "What Are the Signs and Symptoms of a Stroke?". www.nhlbi.nih.gov. March 26, 2014. Archived from the original on 27 February 2015. Retrieved 27 February 2015.
- ↑ PhD, Gary Martin (2009). Palliative Care Nursing: Quality Care to the End of Life, Third Edition. Springer Publishing Company. p. 290. ISBN 978-0-8261-5792-8. Archived from the original on 2017-08-03.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "What Is a Stroke?". www.nhlbi.nih.gov/. March 26, 2014. Archived from the original on 18 February 2015. Retrieved 26 February 2015.
- ↑ 6.0 6.1 6.2 "Who Is at Risk for a Stroke?". www.nhlbi.nih.gov. March 26, 2014. Archived from the original on 27 February 2015. Retrieved 27 February 2015.
- ↑ 7.0 7.1 7.2 Hu, A; Niu, J; Winkelmayer, WC (November 2018). "Oral Anticoagulation in Patients With End-Stage Kidney Disease on Dialysis and Atrial Fibrillation". Seminars in Nephrology. 38 (6): 618–28. doi:10.1016/j.semnephrol.2018.08.006. PMC 6233322. PMID 30413255.
- ↑ 8.0 8.1 8.2 8.3 8.4 "How Is a Stroke Diagnosed?". www.nhlbi.nih.gov. March 26, 2014. Archived from the original on 27 February 2015. Retrieved 27 February 2015.
- ↑ 9.0 9.1 9.2 Yew KS, Cheng E (July 2009). "Acute stroke diagnosis". American Family Physician. 80 (1): 33–40. PMC 2722757. PMID 19621844.
- ↑ 10.0 10.1 GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". The Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ↑ 11.0 11.1 11.2 GBD 2015 Mortality and Causes of Death Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". The Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ↑ 12.0 12.1 12.2 "Types of Stroke". www.nhlbi.nih.gov. March 26, 2014. Archived from the original on 19 March 2015. Retrieved 27 February 2015.
- ↑ Roos, Karen L. (2012). Emergency Neurology. Springer Science & Business Media. p. 360. ISBN 978-0-387-88584-1. Archived from the original on 2017-01-08.
- ↑ Wityk, Robert J.; Llinas, Rafael H. (2007). Stroke. ACP Press. p. 296. ISBN 978-1-930513-70-9. Archived from the original on 2017-01-08.
- ↑ Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, Anderson CS (December 2005). "Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies". Stroke. 36 (12): 2773–80. doi:10.1161/01.STR.0000190838.02954.e8. PMID 16282541.
- ↑ Global Burden of Disease Study 2013 Collaborators (August 2015). "Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013". The Lancet. 386 (9995): 743–800. doi:10.1016/s0140-6736(15)60692-4. PMC 4561509. PMID 26063472.
- ↑ 17.0 17.1 17.2 17.3 17.4 Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. (January 2014). "Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010". The Lancet. 383 (9913): 245–54. doi:10.1016/S0140-6736(13)61953-4. PMC 4181600. PMID 24449944.
- ↑ "Brain Basics: Preventing Stroke". National Institute of Neurological Disorders and Stroke. Archived from the original on 2009-10-08. Retrieved 2009-10-24.
- ↑ World Health Organisation (1978). Cerebrovascular Disorders (Offset Publications). Geneva: World Health Organization. ISBN 978-92-4-170043-6. OCLC 4757533.
- ↑ Kidwell CS, Warach S (December 2003). "Acute ischemic cerebrovascular syndrome: diagnostic criteria". Stroke. 34 (12): 2995–8. doi:10.1161/01.STR.0000098902.69855.A9. PMID 14605325.
- ↑ Shuaib A, Hachinski VC (September 1991). "Mechanisms and management of stroke in the elderly". CMAJ. 145 (5): 433–43. PMC 1335826. PMID 1878825.
- ↑ 22.0 22.1 Stam J (April 2005). "Thrombosis of the cerebral veins and sinuses". The New England Journal of Medicine. 352 (17): 1791–8. doi:10.1056/NEJMra042354. PMID 15858188. Archived from the original on 2020-02-14. Retrieved 2020-07-19.
- ↑ Guercini F, Acciarresi M, Agnelli G, Paciaroni M (April 2008). "Cryptogenic stroke: time to determine aetiology". Journal of Thrombosis and Haemostasis. 6 (4): 549–54. doi:10.1111/j.1538-7836.2008.02903.x. PMID 18208534.
- ↑ Bamford J, Sandercock P, Dennis M, Burn J, Warlow C (June 1991). "Classification and natural history of clinically identifiable subtypes of cerebral infarction". The Lancet. 337 (8756): 1521–6. doi:10.1016/0140-6736(91)93206-O. PMID 1675378. Later publications distinguish between "syndrome" and "infarct", based on evidence from imaging. "Syndrome" may be replaced by "hemorrhage" if imaging demonstrates a bleed. See Internet Stroke Center. "Oxford Stroke Scale". Archived from the original on 2008-10-25. Retrieved 2008-11-14.
- ↑ Bamford JM (2000). "The role of the clinical examination in the subclassification of stroke". Cerebrovascular Diseases. 10 Suppl 4 (4): 2–4. doi:10.1159/000047582. PMID 11070389.
- ↑ Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE (January 1993). "Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment". Stroke. 24 (1): 35–41. doi:10.1161/01.STR.24.1.35. PMID 7678184.[permanent dead link]
- ↑ Osterweil, Neil (2006-12-26). "Methamphetamine induced ischemic strokes". Medpagetoday. Archived from the original on 13 December 2013. Retrieved 24 August 2013.
- ↑ Anonymous (2014-07-29). "Hemorrhagic stroke". National Stroke Association. Archived from the original on 27 June 2016. Retrieved 30 June 2016.
- ↑ Anonymous (6 December 2013). "Types of Stroke". www.cdc.gov. Centers of Disease Control and Prevention. Archived from the original on 27 June 2016. Retrieved 30 June 2016.
- ↑ Al-Shahi Salman R, Labovitz DL, Stapf C (July 2009). "Spontaneous intracerebral haemorrhage". BMJ. 339 (jul24 1): b2586. doi:10.1136/bmj.b2586. PMID 19633038.
- ↑ Goldstein LB, Simel DL (May 2005). "Is this patient having a stroke?". JAMA. 293 (19): 2391–402. doi:10.1001/jama.293.19.2391. PMID 15900010. Archived from the original on 2021-08-29. Retrieved 2020-07-19.
- ↑ Harbison J, Massey A, Barnett L, Hodge D, Ford GA (June 1999). "Rapid ambulance protocol for acute stroke". The Lancet. 353 (9168): 1935. doi:10.1016/S0140-6736(99)00966-6. PMID 10371574.
- ↑ Kidwell CS, Saver JL, Schubert GB, Eckstein M, Starkman S (1998). "Design and retrospective analysis of the Los Angeles Prehospital Stroke Screen (LAPSS)". Prehospital Emergency Care. 2 (4): 267–73. doi:10.1080/10903129808958878. PMID 9799012.
- ↑ Kothari RU, Pancioli A, Liu T, Brott T, Broderick J (April 1999). "Cincinnati Prehospital Stroke Scale: reproducibility and validity". Annals of Emergency Medicine. 33 (4): 373–8. doi:10.1016/S0196-0644(99)70299-4. PMID 10092713.
- ↑ 35.0 35.1 National Institute for Health and Clinical Excellence. Clinical guideline 68: Stroke. London, 2008.
- ↑ Merwick Á, Werring D (May 2014). "Posterior circulation ischaemic stroke". BMJ. 348 (may19 33): g3175. doi:10.1136/bmj.g3175. PMID 24842277.
- ↑ Nor AM, Davis J, Sen B, Shipsey D, Louw SJ, Dyker AG, et al. (November 2005). "The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument". The Lancet. Neurology. 4 (11): 727–34. doi:10.1016/S1474-4422(05)70201-5. PMID 16239179.
- ↑ 38.0 38.1 38.2 38.3 38.4 38.5 O'Sullivan, Susan.B (2007). "Stroke". In O'Sullivan, S.B.; Schmitz, T.J. (eds.). Physical Rehabilitation. Vol. 5. Philadelphia: F.A. Davis Company. p. 719.
- ↑ "Thrombus". MedlinePlus. U.S. National Library of Medicine. Archived from the original on 2016-07-01.
- ↑ "Circle of Willis". The Internet Stroke Center. Archived from the original on 2016-02-05.
- ↑ "Brain anaurysm – Introduction". NHS Choices. 2017-10-19. Archived from the original on 2016-02-08.
- ↑ Fisher CM (December 1968). "The arterial lesions underlying lacunes". Acta Neuropathologica. 12 (1): 1–15. doi:10.1007/BF00685305. PMID 5708546.
- ↑ 43.0 43.1 43.2 43.3 43.4 43.5 43.6 National Institute of Neurological Disorders and Stroke (NINDS) (1999). "Stroke: Hope Through Research". National Institutes of Health. Archived from the original on 2015-10-04.
- ↑ Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. (March 2015). "Short term exposure to air pollution and stroke: systematic review and meta-analysis". BMJ. 350 (mar23 11): h1295. doi:10.1136/bmj.h1295. PMC 4373601. PMID 25810496.
- ↑ 45.0 45.1 Kumar, Vinay (2009). Robbins and Cotran Pathologic Basis of Disease, Professional Edition (8th ed.). Philadelphia: Elsevier. ISBN 978-1-4377-0792-2.
- ↑ Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ (November 2005). "An evidence-based causative classification system for acute ischemic stroke". Annals of Neurology. 58 (5): 688–97. doi:10.1002/ana.20617. PMID 16240340.
- ↑ Hackam DG (May 2016). "Prognosis of Asymptomatic Carotid Artery Occlusion: Systematic Review and Meta-Analysis". Stroke. 47 (5): 1253–7. doi:10.1161/strokeaha.116.012760. PMID 27073237.
- ↑ Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ (April 2017). "Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update". Stroke. 48 (4): 867–872. doi:10.1161/STROKEAHA.116.016414. PMID 28265016.
- ↑ Strandgaard, Svend (October 1996). "Hypertension". Journal of Hypertension. 14 (3): S23–S27. doi:10.1097/00004872-199610003-00005. PMID 9120662.
- ↑ Harrigan, Mark R.; Deveikis, John P. (2012). Handbook of Cerebrovascular Disease and Neurointerventional Technique. Springer Science & Business Media. p. 692. ISBN 978-1-61779-945-7. Archived from the original on 2017-01-09.
- ↑ Miwa K, Hoshi T, Hougaku H, Tanaka M, Furukado S, Abe Y, et al. (2010). "Silent cerebral infarction is associated with incident stroke and TIA independent of carotid intima-media thickness". Internal Medicine. 49 (9): 817–22. doi:10.2169/internalmedicine.49.3211. PMID 20453400.
- ↑ 52.0 52.1 Herderscheê D, Hijdra A, Algra A, Koudstaal PJ, Kappelle LJ, van Gijn J (September 1992). "Silent stroke in patients with transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group". Stroke. 23 (9): 1220–4. doi:10.1161/01.STR.23.9.1220. PMID 1519274.[permanent dead link]
- ↑ Leary MC, Saver JL (2003). "Annual incidence of first silent stroke in the United States: a preliminary estimate". Cerebrovascular Diseases. 16 (3): 280–5. doi:10.1159/000071128. PMID 12865617.
- ↑ Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM (January 2002). "Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study". Stroke. 33 (1): 21–5. doi:10.1161/hs0102.101629. PMID 11779883.
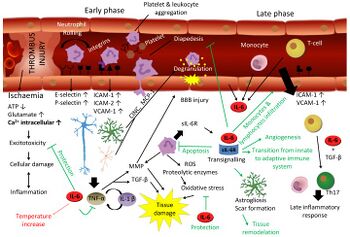
- ↑ Erta, María; Quintana, Albert; Hidalgo, Juan (2012). "Interleukin-6, a major cytokine in the central nervous system". International Journal of Biological Sciences. 8 (9): 1254–1266. doi:10.7150/ijbs.4679. ISSN 1449-2288. Retrieved 27 July 2023. To some extent the response of the brain to trauma and stroke is similar. Stroke may be caused by an embolus/thrombus occlusion, an hemorrhage or a vasospasm, resulting in ischemia. Hypoxia initiates a biochemical cascade leading towards cell death, involving excitotoxicity, oxidative stress and apoptosis in which IL-6 has a protective effect.
- ↑ Deb P, Sharma S, Hassan KM (June 2010). "Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis". Pathophysiology. 17 (3): 197–218. doi:10.1016/j.pathophys.2009.12.001. PMID 20074922.
- ↑ Richard S. Snell (2006). Clinical neuroanatomy, 6. ed. Lippincott Williams & Wilkins, Philadelphia. pp. 478–85. ISBN 978-963-226-293-2.
- ↑ Brunner and Suddarth's Textbook on Medical-Surgical Nursing, 11th Edition
- ↑ Kristián T, Siesjö BK (1996). "Calcium-related damage in ischemia". Life Sciences. 59 (5–6): 357–67. doi:10.1016/0024-3205(96)00314-1. PMID 8761323.
- ↑ Chan PH (January 2001). "Reactive oxygen radicals in signaling and damage in the ischemic brain". Journal of Cerebral Blood Flow and Metabolism. 21 (1): 2–14. doi:10.1097/00004647-200101000-00002. PMID 11149664.
- ↑ Longo, Dan L.; et al. (2012). Harrison's Principles of Internal Medicine (18th ed.). New York: McGraw-Hill. p. 370. ISBN 978-0-07-174889-6.
- ↑ 62.0 62.1 Wang J (December 2010). "Preclinical and clinical research on inflammation after intracerebral hemorrhage". Progress in Neurobiology. 92 (4): 463–77. doi:10.1016/j.pneurobio.2010.08.001. PMC 2991407. PMID 20713126.
- ↑ Hill MD (November 2005). "Diagnostic biomarkers for stroke: a stroke neurologist's perspective". Clinical Chemistry. 51 (11): 2001–2. doi:10.1373/clinchem.2005.056382. PMID 16244286.
- ↑ Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. (January 2007). "Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison". The Lancet. 369 (9558): 293–8. doi:10.1016/S0140-6736(07)60151-2. PMC 1859855. PMID 17258669.
- ↑ Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, et al. (October 2004). "Comparison of MRI and CT for detection of acute intracerebral hemorrhage". JAMA. 292 (15): 1823–30. doi:10.1001/jama.292.15.1823. PMID 15494579.
- ↑ 66.0 66.1 Wilson D, Adams ME, Robertson F, Murphy M, Werring DJ (May 2015). "Investigating intracerebral haemorrhage". BMJ. 350 (may20 10): h2484. doi:10.1136/bmj.h2484. PMID 25994363.
- ↑ 67.0 67.1 67.2 67.3 67.4 Bakradze E, Liberman AL (February 2018). "Diagnostic Error in Stroke-Reasons and Proposed Solutions". Current Atherosclerosis Reports. 20 (2): 11. doi:10.1007/s11883-018-0712-3. PMID 29441421.
- ↑ Dupre CM, Libman R, Dupre SI, Katz JM, Rybinnik I, Kwiatkowski T (February 2014) [Available online 15 August 2013]. "Stroke chameleons". Journal of Stroke and Cerebrovascular Diseases. 23 (2): 374–8. doi:10.1016/j.jstrokecerebrovasdis.2013.07.015. PMID 23954604.
- ↑ 69.0 69.1 Straus SE, Majumdar SR, McAlister FA (September 2002). "New evidence for stroke prevention: scientific review". JAMA. 288 (11): 1388–95. doi:10.1001/jama.288.11.1388. PMID 12234233.
- ↑ Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. (June 2006). "Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline". Stroke. 37 (6): 1583–633. doi:10.1161/01.STR.0000223048.70103.F1. PMID 16675728.
- ↑ Center for Drug Evaluation and Research. "Information for Consumers (Drugs) - Use of Aspirin for Primary Prevention of Heart Attack and Stroke". www.fda.gov. Archived from the original on 2015-11-17. Retrieved 2015-11-16.
- ↑ 72.0 72.1 72.2 NPS Prescribing Practice Review 44: Antiplatelets and anticoagulants in stroke prevention (2009). Available at nps.org.au Archived 2012-04-07 at the Wayback Machine
- ↑ 73.0 73.1 Antithrombotic Trialists' Collaboration (January 2002). "Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients". BMJ. 324 (7329): 71–86. doi:10.1136/bmj.324.7329.71. PMC 64503. PMID 11786451.
- ↑ Jonas DE, Feltner C, Amick HR, Sheridan S, Zheng ZJ, Watford DJ, et al. (September 2014). "Screening for asymptomatic carotid artery stenosis: a systematic review and meta-analysis for the U.S. Preventive Services Task Force". Annals of Internal Medicine. 161 (5): 336–46. doi:10.7326/M14-0530. PMID 25004169.
- ↑ Medical Research Council Working Party (July 1985). "MRC trial of treatment of mild hypertension: principal results". British Medical Journal. 291 (6488): 97–104. doi:10.1136/bmj.291.6488.97. PMC 1416260. PMID 2861880.
- ↑ Thomson R (August 2009). "Evidence based implementation of complex interventions". BMJ. 339: b3124. doi:10.1136/bmj.b3124. PMID 19675081.
- ↑ Hankey GJ (August 1999). "Smoking and risk of stroke". Journal of Cardiovascular Risk. 6 (4): 207–11. doi:10.1177/204748739900600403. PMID 10501270.
- ↑ Wannamethee SG, Shaper AG, Whincup PH, Walker M (July 1995). "Smoking cessation and the risk of stroke in middle-aged men". JAMA. 274 (2): 155–60. doi:10.1001/jama.274.2.155. PMID 7596004.
- ↑ Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J, Lewis BL (February 2003). "Alcohol consumption and risk of stroke: a meta-analysis". JAMA. 289 (5): 579–88. doi:10.1001/jama.289.5.579. PMID 12578491. Archived from the original on 2021-08-29. Retrieved 2020-07-19.
- ↑ Sloan MA, Kittner SJ, Rigamonti D, Price TR (September 1991). "Occurrence of stroke associated with use/abuse of drugs". Neurology. 41 (9): 1358–64. doi:10.1212/WNL.41.9.1358. PMID 1891081.
- ↑ Larsson SC, Virtamo J, Wolk A (August 2011). "Red meat consumption and risk of stroke in Swedish men". The American Journal of Clinical Nutrition. 94 (2): 417–21. doi:10.3945/ajcn.111.015115. PMID 21653800.
- ↑ "Stroke Risk Factors". American Heart Association. 2007. Archived from the original on November 18, 2010. Retrieved January 22, 2007.
- ↑ Hackshaw A, Morris JK, Boniface S, Tang JL, Milenković D (January 2018). "Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports". BMJ. 360: j5855. doi:10.1136/bmj.j5855. PMC 5781309. PMID 29367388.
- ↑ Gorelick PB (1987). "Alcohol and stroke". Stroke. 18 (1): 268–71. doi:10.1161/01.STR.18.1.268. PMID 3810763.[permanent dead link]
- ↑ Longo, Dan L.; et al., eds. (2012). Harrison's principles of internal medicine (18th ed.). New York: McGraw-Hill. pp. Chapter 370. ISBN 978-0-07-174889-6.
- ↑ Westover AN, McBride S, Haley RW (April 2007). "Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients". Archives of General Psychiatry. 64 (4): 495–502. doi:10.1001/archpsyc.64.4.495. PMID 17404126.
- ↑ Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T (October 2009). "Migraine and cardiovascular disease: systematic review and meta-analysis". BMJ. 339 (oct27 1): b3914. doi:10.1136/bmj.b3914. PMC 2768778. PMID 19861375.
- ↑ Kurth T, Chabriat H, Bousser MG (January 2012). "Migraine and stroke: a complex association with clinical implications". The Lancet. Neurology. 11 (1): 92–100. doi:10.1016/S1474-4422(11)70266-6. PMID 22172624.
- ↑ Ciaccio EJ, Lewis SK, Biviano AB, Iyer V, Garan H, Green PH (August 2017). "Cardiovascular involvement in celiac disease". World Journal of Cardiology (Review). 9 (8): 652–666. doi:10.4330/wjc.v9.i8.652. PMC 5583538. PMID 28932354.
- ↑ Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. (August 2016). "Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013". BMJ. 354: i3857. doi:10.1136/bmj.i3857. PMC 4979358. PMID 27510511.
- ↑ Ezekowitz JA, Straus SE, Majumdar SR, McAlister FA (December 2003). "Stroke: strategies for primary prevention". American Family Physician. 68 (12): 2379–86. PMID 14705756.
- ↑ 92.0 92.1 92.2 92.3 Ederle J, Brown MM (October 2006). "The evidence for medicine versus surgery for carotid stenosis". European Journal of Radiology. 60 (1): 3–7. doi:10.1016/j.ejrad.2006.05.021. PMID 16920313.
- ↑ Whisnant JP (February 1996). "Effectiveness versus efficacy of treatment of hypertension for stroke prevention". Neurology. 46 (2): 301–7. doi:10.1212/WNL.46.2.301. PMID 8614485.
- ↑ Law MR, Morris JK, Wald NJ (May 2009). "Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies". BMJ. 338: b1665. doi:10.1136/bmj.b1665. PMC 2684577. PMID 19454737.
- ↑ Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS (May 2003). "Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis". JAMA. 289 (19): 2534–44. doi:10.1001/jama.289.19.2534. PMID 12759325.
- ↑ 96.0 96.1 "Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Prospective studies collaboration". The Lancet. 346 (8991–8992): 1647–53. 1995. doi:10.1016/S0140-6736(95)92836-7. PMID 8551820.
- ↑ Gueyffier F, Boissel JP, Boutitie F, Pocock S, Coope J, Cutler J, et al. (December 1997). "Effect of antihypertensive treatment in patients having already suffered from stroke. Gathering the evidence. The INDANA (INdividual Data ANalysis of Antihypertensive intervention trials) Project Collaborators". Stroke. 28 (12): 2557–62. doi:10.1161/01.STR.28.12.2557. PMID 9412649.
- ↑ Gueyffier F, Bulpitt C, Boissel JP, Schron E, Ekbom T, Fagard R, et al. (March 1999). "Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. INDANA Group". The Lancet. 353 (9155): 793–6. doi:10.1016/S0140-6736(98)08127-6. PMID 10459960. Archived from the original on 2020-08-07. Retrieved 2020-07-19.
- ↑ Staessen JA, Gasowski J, Wang JG, Thijs L, Den Hond E, Boissel JP, et al. (March 2000). "Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials". The Lancet. 355 (9207): 865–72. doi:10.1016/S0140-6736(99)07330-4. PMID 10752701.
- ↑ Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. (May 2008). "Treatment of hypertension in patients 80 years of age or older" (PDF). The New England Journal of Medicine. 358 (18): 1887–98. doi:10.1056/NEJMoa0801369. PMID 18378519. Archived (PDF) from the original on 2019-04-12. Retrieved 2020-07-19.
- ↑ Neal B, MacMahon S, Chapman N (December 2000). "Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration". The Lancet. 356 (9246): 1955–64. doi:10.1016/S0140-6736(00)03307-9. PMID 11130523.
- ↑ The Allhat Officers And Coordinators For The Allhat Collaborative Research Group (December 2002). "Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)". JAMA. 288 (23): 2981–97. doi:10.1001/jama.288.23.2981. PMID 12479763.
- ↑ De Lima LG, Saconato H, Atallah AN, da Silva EM (October 2014). "Beta-blockers for preventing stroke recurrence". The Cochrane Database of Systematic Reviews. 10 (10): CD007890. doi:10.1002/14651858.CD007890.pub3. PMID 25317988.
- ↑ Iso H, Jacobs DR, Wentworth D, Neaton JD, Cohen JD (April 1989). "Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial". The New England Journal of Medicine. 320 (14): 904–10. doi:10.1056/NEJM198904063201405. PMID 2619783.
- ↑ 105.0 105.1 O'Regan C, Wu P, Arora P, Perri D, Mills EJ (January 2008). "Statin therapy in stroke prevention: a meta-analysis involving 121,000 patients". The American Journal of Medicine. 121 (1): 24–33. doi:10.1016/j.amjmed.2007.06.033. PMID 18187070.
- ↑ Hebert PR, Gaziano JM, Hennekens CH (January 1995). "An overview of trials of cholesterol lowering and risk of stroke". Archives of Internal Medicine. 155 (1): 50–5. doi:10.1001/archinte.155.1.50. PMID 7802520.
- ↑ "Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group". The Lancet. 352 (9131): 837–53. September 1998. doi:10.1016/S0140-6736(98)07019-6. PMID 9742976.
- ↑ Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. (October 2005). "Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial". The Lancet. 366 (9493): 1279–89. doi:10.1016/S0140-6736(05)67528-9. PMID 16214598.
- ↑ Johnson ES, Lanes SF, Wentworth CE, Satterfield MH, Abebe BL, Dicker LW (June 1999). "A metaregression analysis of the dose-response effect of aspirin on stroke". Archives of Internal Medicine. 159 (11): 1248–53. doi:10.1001/archinte.159.11.1248. PMID 10371234.
- ↑ 110.0 110.1 Sudlow CL, Mason G, Maurice JB, Wedderburn CJ, Hankey GJ (October 2009). "Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients". The Cochrane Database of Systematic Reviews (4): CD001246. doi:10.1002/14651858.CD001246.pub2. PMC 7055203. PMID 19821273. Archived from the original on 2021-08-29. Retrieved 2020-07-19.
- ↑ Hao Q, Tampi M, O'Donnell M, Foroutan F, Siemieniuk RA, Guyatt G (December 2018). "Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis". BMJ. 363: k5108. doi:10.1136/bmj.k5108. PMC 6298178. PMID 30563866.
- ↑ Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A (May 2006). "Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial". The Lancet. 367 (9523): 1665–73. doi:10.1016/S0140-6736(06)68734-5. PMID 16714187.
- ↑ Wolf PA, Abbott RD, Kannel WB (September 1987). "Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study". Archives of Internal Medicine. 147 (9): 1561–4. doi:10.1001/archinte.147.9.1561. PMID 3632164.
- ↑ Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. (August 2006). "ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society". Circulation. 114 (7): e257-354. doi:10.1161/CIRCULATIONAHA.106.177292. PMID 16908781.
- ↑ Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A (February 2007). "Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial". The Lancet. Neurology. 6 (2): 115–24. doi:10.1016/S1474-4422(06)70685-8. PMID 17239798.
- ↑ Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA (March 2000). "Aspirin for the primary prevention of stroke and other major vascular events: meta-analysis and hypotheses". Archives of Neurology. 57 (3): 326–32. doi:10.1001/archneur.57.3.326. PMID 10714657.
- ↑ Bartolucci AA, Howard G (September 2006). "Meta-analysis of data from the six primary prevention trials of cardiovascular events using aspirin". The American Journal of Cardiology. 98 (6): 746–50. doi:10.1016/j.amjcard.2006.04.012. PMID 16950176.
- ↑ Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL (January 2006). "Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials". JAMA. 295 (3): 306–13. doi:10.1001/jama.295.3.306. PMID 16418466.
- ↑ Yerman T, Gan WQ, Sin DD (October 2007). "The influence of gender on the effects of aspirin in preventing myocardial infarction". BMC Medicine. 5: 29. doi:10.1186/1741-7015-5-29. PMC 2131749. PMID 17949479.
- ↑ Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. (January 2003). "Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis". The Lancet. 361 (9352): 107–16. doi:10.1016/S0140-6736(03)12228-3. PMID 12531577.
- ↑ Ringleb PA, Chatellier G, Hacke W, Favre JP, Bartoli JM, Eckstein HH, Mas JL (February 2008). "Safety of endovascular treatment of carotid artery stenosis compared with surgical treatment: a meta-analysis". Journal of Vascular Surgery. 47 (2): 350–5. doi:10.1016/j.jvs.2007.10.035. PMID 18241759.
- ↑ Müller, Mandy D.; Lyrer, Philippe; Brown, Martin M.; Bonati, Leo H. (25 February 2020). "Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis". The Cochrane Database of Systematic Reviews. 2: CD000515. doi:10.1002/14651858.CD000515.pub5. ISSN 1469-493X. PMC 7041119. PMID 32096559.
- ↑ Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ (March 2004). "Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery". The Lancet. 363 (9413): 915–24. doi:10.1016/S0140-6736(04)15785-1. PMID 15043958.
- ↑ Fairhead JF, Mehta Z, Rothwell PM (August 2005). "Population-based study of delays in carotid imaging and surgery and the risk of recurrent stroke". Neurology. 65 (3): 371–5. doi:10.1212/01.wnl.0000170368.82460.b4. PMID 16087900.
- ↑ U.S. Preventive Services Task Force (December 2007). "Screening for carotid artery stenosis: U.S. Preventive Services Task Force recommendation statement". Annals of Internal Medicine. 147 (12): 854–9. doi:10.7326/0003-4819-147-12-200712180-00005. PMID 18087056.
- ↑ Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D (May 2004). "Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial". The Lancet. 363 (9420): 1491–502. doi:10.1016/S0140-6736(04)16146-1. PMID 15135594.
- ↑ Chambers BR, Donnan GA (October 2005). "Carotid endarterectomy for asymptomatic carotid stenosis". The Cochrane Database of Systematic Reviews (4): CD001923. doi:10.1002/14651858.CD001923.pub2. PMC 6669257. PMID 16235289.
- ↑ Spence JD (September 2006). "Nutrition and stroke prevention". Stroke. 37 (9): 2430–5. doi:10.1161/01.STR.0000236633.40160.ee. PMID 16873712.
- ↑ Zhou YH, Tang JY, Wu MJ, Lu J, Wei X, Qin YY, et al. (2011). "Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis". PLOS ONE. 6 (9): e25142. Bibcode:2011PLoSO...625142Z. doi:10.1371/journal.pone.0025142. PMC 3182189. PMID 21980387.
- ↑ Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, et al. (October 2010). "Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals". Archives of Internal Medicine. 170 (18): 1622–31. doi:10.1001/archinternmed.2010.348. PMID 20937919.
- ↑ Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. (May 2014). "Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association". Stroke. 45 (5): 1545–88. doi:10.1161/01.str.0000442009.06663.48. PMID 24503673.
- ↑ 132.0 132.1 Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. (July 2014). "Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association". Stroke. 45 (7): 2160–236. doi:10.1161/STR.0000000000000024. PMID 24788967.
- ↑ Hart RG, Pearce LA, Aguilar MI (June 2007). "Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation". Annals of Internal Medicine. 146 (12): 857–67. doi:10.7326/0003-4819-146-12-200706190-00007. PMID 17577005.
- ↑ Paciaroni M, Agnelli G, Micheli S, Caso V (February 2007). "Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials". Stroke. 38 (2): 423–30. doi:10.1161/01.STR.0000254600.92975.1f. PMID 17204681.ACP JC synopsis Archived 2012-11-14 at the Wayback Machine
- ↑ Armstrong MJ, Gronseth G, Anderson DC, Biller J, Cucchiara B, Dafer R, et al. (May 2013). "Summary of evidence-based guideline: periprocedural management of antithrombotic medications in patients with ischemic cerebrovascular disease: report of the Guideline Development Subcommittee of the American Academy of Neurology". Neurology. 80 (22): 2065–9. doi:10.1212/WNL.0b013e318294b32d. PMC 3716407. PMID 23713086.
- ↑ Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z (July 2016). "Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials". The Lancet. 388 (10042): 365–375. doi:10.1016/S0140-6736(16)30468-8. PMC 5321490. PMID 27209146.
- ↑ Gomez CR (1993). "Editorial: Time is brain!". Journal of Stroke and Cerebrovascular Diseases. 3 (1): 1–2. doi:10.1016/S1052-3057(10)80125-9. PMID 26487071.
- ↑ Saver JL (January 2006). "Time is brain--quantified". Stroke. 37 (1): 263–6. doi:10.1161/01.STR.0000196957.55928.ab. PMID 16339467.
- ↑ Bálint, A; Tornyos, D; El Alaoui El Abdallaoui, O; Kupó, P; Komócsi, A (August 2021). "Network Meta-Analysis of Ticagrelor for Stroke Prevention in Patients at High Risk for Cardiovascular or Cerebrovascular Events". Stroke. 52 (9): 2809–2816. doi:10.1161/STROKEAHA.120.032670. PMID 34162232.
- ↑ Trifan, G; Gorelick, PB; Testai, FD (22 June 2021). "Efficacy and Safety of Using Dual Versus Monotherapy Antiplatelet Agents in Secondary Stroke Prevention: Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials". Circulation. 143 (25): 2441–2453. doi:10.1161/CIRCULATIONAHA.121.053782. PMID 33926204.
- ↑ Bellolio MF, Gilmore RM, Ganti L (January 2014). "Insulin for glycaemic control in acute ischaemic stroke". The Cochrane Database of Systematic Reviews. 1 (1): CD005346. doi:10.1002/14651858.CD005346.pub4. PMID 24453023.
- ↑ Bath PM, Krishnan K (October 2014). "Interventions for deliberately altering blood pressure in acute stroke" (PDF). The Cochrane Database of Systematic Reviews. 10 (10): CD000039. doi:10.1002/14651858.CD000039.pub3. PMC 7052738. PMID 25353321. Archived from the original (PDF) on 2019-04-12. Retrieved 2020-07-19.
- ↑ Lee M, Ovbiagele B, Hong KS, Wu YL, Lee JE, Rao NM, et al. (July 2015). "Effect of Blood Pressure Lowering in Early Ischemic Stroke: Meta-Analysis". Stroke. 46 (7): 1883–9. doi:10.1161/STROKEAHA.115.009552. PMID 26022636.
- ↑ Ziganshina, Liliya Eugenevna; Abakumova, Tatyana; Hoyle, Charles Hv (14 July 2020). "Cerebrolysin for acute ischaemic stroke". The Cochrane Database of Systematic Reviews. 7: CD007026. doi:10.1002/14651858.CD007026.pub6. ISSN 1469-493X. PMC 7387239. PMID 32662068. Archived from the original on 29 August 2021. Retrieved 4 September 2020.
- ↑ 145.0 145.1 145.2 Wardlaw JM, Murray V, Berge E, del Zoppo GJ (July 2014). "Thrombolysis for acute ischaemic stroke". The Cochrane Database of Systematic Reviews. 7 (7): CD000213. doi:10.1002/14651858.CD000213.pub3. PMC 4153726. PMID 25072528.
- ↑ 146.0 146.1 Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. (November 2014). "Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials". The Lancet. 384 (9958): 1929–35. doi:10.1016/S0140-6736(14)60584-5. PMC 4441266. PMID 25106063.
- ↑ 147.0 147.1 "Thrombolytics for acute stroke". Dynamed. Sep 15, 2014. Archived from the original on 7 January 2016. Retrieved 4 October 2014.
at 3–4.5 hours after stroke onset t-PA increases risk of symptomatic intracranial hemorrhage but effect on functional outcomes is inconsistent
- ↑ Alper BS, Malone-Moses M, McLellan JS, Prasad K, Manheimer E (March 2015). "Thrombolysis in acute ischaemic stroke: time for a rethink?". BMJ. 350 (h1075): h1075. doi:10.1136/bmj.h1075. PMID 25786912. Archived from the original on 2021-08-29. Retrieved 2020-07-19.
- ↑ "Canadian Association of Emergency Physicians Position Statement on Acute Ischemic Stroke" (PDF). caep.ca. March 2015. Archived from the original (PDF) on 2015-09-18. Retrieved 7 April 2015.
- ↑ "10 points to remember". American College of Cardiology. 2018. Archived from the original on March 28, 2020. Retrieved March 27, 2020.
- ↑ Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G (June 2012). "Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis". The Lancet. 379 (9834): 2364–72. doi:10.1016/S0140-6736(12)60738-7. PMC 3386494. PMID 22632907.
- ↑ Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J (November 2012). "Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies". Stroke. 43 (11): 2904–9. doi:10.1161/STROKEAHA.112.665331. PMID 22996959.
- ↑ Campbell BC, Ma H, Ringleb PA, Parsons MW, Churilov L, Bendszus M, et al. (July 2019). "Extending thrombolysis to 4·5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data". The Lancet. 394 (10193): 139–147. doi:10.1016/S0140-6736(19)31053-0. hdl:10138/312914. PMID 31128925.
- ↑ Thomalla, G; Boutitie, F; Ma, H; Koga, M; Ringleb, P; Schwamm, LH; Wu, O; Bendszus, M; Bladin, CF; Campbell, BCV; Cheng, B; Churilov, L; Ebinger, M; Endres, M; Fiebach, JB; Fukuda-Doi, M; Inoue, M; Kleinig, TJ; Latour, LL; Lemmens, R; Levi, CR; Leys, D; Miwa, K; Molina, CA; Muir, KW; Nighoghossian, N; Parsons, MW; Pedraza, S; Schellinger, PD; Schwab, S; Simonsen, CZ; Song, SS; Thijs, V; Toni, D; Hsu, CY; Wahlgren, N; Yamamoto, H; Yassi, N; Yoshimura, S; Warach, S; Hacke, W; Toyoda, K; Donnan, GA; Davis, SM; Gerloff, C; Evaluation of unknown Onset Stroke thrombolysis trials (EOS), investigators. (14 November 2020). "Intravenous alteplase for stroke with unknown time of onset guided by advanced imaging: systematic review and meta-analysis of individual patient data". Lancet (London, England). 396 (10262): 1574–1584. doi:10.1016/S0140-6736(20)32163-2. PMID 33176180.
- ↑ The National Institute Of Neurological Disorders And Stroke Rt-Pa Stroke Study Group (December 1995). "Tissue plasminogen activator for acute ischemic stroke". The New England Journal of Medicine. 333 (24): 1581–7. doi:10.1056/NEJM199512143332401. PMID 7477192.
- ↑ Wardlaw JM, Murray V, Berge E, del Zoppo GJ (July 2014). "Thrombolysis for acute ischaemic stroke". The Cochrane Database of Systematic Reviews. 7 (7): CD000213. doi:10.1002/14651858.CD000213.pub3. PMC 4153726. PMID 25072528.
- ↑ Dubinsky R, Lai SM (June 2006). "Mortality of stroke patients treated with thrombolysis: analysis of nationwide inpatient sample". Neurology. 66 (11): 1742–4. doi:10.1212/01.wnl.0000218306.35681.38. PMID 16769953.
- ↑ "Position Statement on the Use of Intravenous Thrombolytic Therapy in the Treatment of Stroke". American Academy of Emergency Medicine. Archived from the original on 2006-10-04. Retrieved 2008-01-25.
- ↑ Chapman, Sherita N; Mehndiratta, Prachi; Johansen, Michelle C; McMurry, Timothy L; Johnston, Karen C; Southerland, Andrew M (2014-02-24). "Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke". Vascular Health and Risk Management. 10: 75–87. doi:10.2147/VHRM.S39213. ISSN 1176-6344. PMC 3938499. PMID 24591838.
- ↑ American College of Emergency Physicians (2013). "Clinical Policy: Use of Intravenous tPA for the Management of Acute Ischemic Stroke in the Emergency Department". Annals of Emergency Medicine. 61 (2): 225–243. doi:10.1016/j.annemergmed.2012.11.005. ISSN 0196-0644. PMID 23331647. Archived from the original on 2021-08-29. Retrieved 2020-07-19.
- ↑ Kolata, Gina (2018-03-26). "For Many Strokes, There's an Effective Treatment. Why Aren't Some Doctors Offering It?". The New York Times. ISSN 0362-4331. Archived from the original on 2020-03-28. Retrieved 2020-03-28.
- ↑ "The Case Against Thrombolytic Therapy in Stroke". Medscape. Archived from the original on 2020-03-28. Retrieved 2020-03-28.
- ↑ Lee M, Hong KS, Saver JL (May 2010). "Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials". Stroke. 41 (5): 932–7. doi:10.1161/STROKEAHA.109.574335. PMID 20360549.
- ↑ Sardar P, Chatterjee S, Giri J, Kundu A, Tandar A, Sen P, et al. (September 2015). "Endovascular therapy for acute ischaemic stroke: a systematic review and meta-analysis of randomized trials". European Heart Journal. 36 (35): 2373–80. doi:10.1093/eurheartj/ehv270. PMID 26071599.
- ↑ Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. (September 2016). "Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis". JAMA. 316 (12): 1279–88. doi:10.1001/jama.2016.13647. PMID 27673305.
- ↑ Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. (April 2016). "Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials". The Lancet. 387 (10029): 1723–31. doi:10.1016/s0140-6736(16)00163-x. PMID 26898852.
- ↑ Mistry EA, Mistry AM, Nakawah MO, Chitale RV, James RF, Volpi JJ, Fusco MR (September 2017). "Mechanical Thrombectomy Outcomes With and Without Intravenous Thrombolysis in Stroke Patients: A Meta-Analysis". Stroke. 48 (9): 2450–2456. doi:10.1161/STROKEAHA.117.017320. PMID 28747462.
- ↑ Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. (March 2018). "2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association". Stroke. 49 (3): e46–e110. doi:10.1161/STR.0000000000000158. PMID 29367334.
- ↑ Simard JM, Sahuquillo J, Sheth KN, Kahle KT, Walcott BP (April 2011). "Managing malignant cerebral infarction". Current Treatment Options in Neurology. 13 (2): 217–29. doi:10.1007/s11940-010-0110-9. PMC 3243953. PMID 21190097.
- ↑ Vespa PM, Martin N, Zuccarello M, Awad I, Hanley DF (June 2013). "Surgical trials in intracerebral hemorrhage". Stroke. 44 (6 Suppl 1): S79-82. doi:10.1161/STROKEAHA.113.001494. PMC 6778724. PMID 23709739.
- ↑ Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G (2013). "European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage" (PDF). Cerebrovascular Diseases. 35 (2): 93–112. doi:10.1159/000346087. PMID 23406828. Archived from the original on 2021-08-29. Retrieved 2020-07-19.
- ↑ Langhorne, Peter; Ramachandra, Samantha; Stroke Unit Trialists' Collaboration (23 April 2020). "Organised inpatient (stroke unit) care for stroke: network meta-analysis". The Cochrane Database of Systematic Reviews. 4: CD000197. doi:10.1002/14651858.CD000197.pub4. ISSN 1469-493X. PMC 7197653. PMID 32324916.
- ↑ Clarke, David J. (May 2014). "Nursing practice in stroke rehabilitation: systematic review and meta-ethnography". Journal of Clinical Nursing. 23 (9–10): 1201–1226. doi:10.1111/jocn.12334. ISSN 1365-2702. PMID 24102924. Archived from the original on 2019-06-07. Retrieved 2020-09-04.
- ↑ Duffy, Laura; Gajree, Shelley; Langhorne, Peter; Stott, David J.; Quinn, Terence J. (February 2013). "Reliability (inter-rater agreement) of the Barthel Index for assessment of stroke survivors: systematic review and meta-analysis". Stroke. 44 (2): 462–468. doi:10.1161/STROKEAHA.112.678615. ISSN 1524-4628. PMID 23299497. Archived from the original on 2021-08-29. Retrieved 2020-09-04.
- ↑ O'Sullivan 2007, pp. 471, 484, 737, 740
- ↑ 176.0 176.1 Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. (April 2014). Cochrane Stroke Group (ed.). "Physical rehabilitation approaches for the recovery of function and mobility following stroke". The Cochrane Database of Systematic Reviews (4): CD001920. doi:10.1002/14651858.CD001920.pub3. PMC 6465059. PMID 24756870.
- ↑ 177.0 177.1 Thomas, Lois H.; Coupe, Jacqueline; Cross, Lucy D.; Tan, Aidan L.; Watkins, Caroline L. (February 2019). "Interventions for treating urinary incontinence after stroke in adults". The Cochrane Database of Systematic Reviews. 2: CD004462. doi:10.1002/14651858.CD004462.pub4. ISSN 1469-493X. PMC 6355973. PMID 30706461.
- ↑ 178.0 178.1 Stratton, Hezekiah; Sansom, Joshua; Brown-Major, Anita; Anderson, Paul; Ng, Louisa (2020-05-01). "Interventions for sexual dysfunction following stroke". The Cochrane Database of Systematic Reviews. 5: CD011189. doi:10.1002/14651858.CD011189.pub2. ISSN 1469-493X. PMC 7197697. PMID 32356377. Archived from the original on 2021-08-29. Retrieved 2020-09-04.
- ↑ Mackenzie C (April 2011). "Dysarthria in stroke: a narrative review of its description and the outcome of intervention". International Journal of Speech-Language Pathology. 13 (2): 125–36. doi:10.3109/17549507.2011.524940. PMID 21480809. Archived from the original on 2020-08-07. Retrieved 2020-07-19.
- ↑ West C, Hesketh A, Vail A, Bowen A (October 2005). "Interventions for apraxia of speech following stroke". The Cochrane Database of Systematic Reviews (4): CD004298. doi:10.1002/14651858.CD004298.pub2. PMID 16235357.
- ↑ Brady MC, Kelly H, Godwin J, Enderby P, Campbell P (June 2016). "Speech and language therapy for aphasia following stroke". The Cochrane Database of Systematic Reviews. 2016 (6): CD000425. doi:10.1002/14651858.CD000425.pub4. hdl:1893/26112. PMID 27245310.
- ↑ Bath, Philip M.; Lee, Han Sean; Everton, Lisa F. (30 October 2018). "Swallowing therapy for dysphagia in acute and subacute stroke". The Cochrane Database of Systematic Reviews. 10: CD000323. doi:10.1002/14651858.CD000323.pub3. ISSN 1469-493X. PMC 6516809. PMID 30376602.
- ↑ "NHS Scotland – SHOW" (PDF). Archived (PDF) from the original on 2013-05-16. Retrieved 2012-11-09.
- ↑ 184.0 184.1 184.2 184.3 Saunders, David H.; Sanderson, Mark; Hayes, Sara; Johnson, Liam; Kramer, Sharon; Carter, Daniel D.; Jarvis, Hannah; Brazzelli, Miriam; Mead, Gillian E. (20 March 2020). "Physical fitness training for stroke patients". The Cochrane Database of Systematic Reviews. 3: CD003316. doi:10.1002/14651858.CD003316.pub7. ISSN 1469-493X. PMC 7083515. PMID 32196635.
- ↑ Institute for Quality and Efficiency in Health Care (IQWiG). "After a stroke: Does fitness training improve overall health and mobility?". Informed Health Online. Institute for Quality and Efficiency in Health Care (IQWiG). Archived from the original on 29 August 2021. Retrieved 20 June 2013.
- ↑ Barclay RE, Stevenson TJ, Poluha W, Ripat J, Nett C, Srikesavan CS (March 2015). "Interventions for improving community ambulation in individuals with stroke". The Cochrane Database of Systematic Reviews. Art. No.: CD010200 (3): CD010200. doi:10.1002/14651858.CD010200.pub2. PMC 6465042. PMID 25767912.
- ↑ 187.0 187.1 Lange B, Flynn S, Rizzo A (2009). "Initial usability assessment of off-the-shelf video game consoles for clinical game-based motor rehabilitation". Physical Therapy Reviews. 14 (5): 355–62. doi:10.1179/108331909X12488667117258. Archived from the original on 2021-08-29. Retrieved 2020-07-19.
- ↑ 188.0 188.1 Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M (November 2017). Cochrane Stroke Group (ed.). "Virtual reality for stroke rehabilitation". The Cochrane Database of Systematic Reviews. 11: CD008349. doi:10.1002/14651858.CD008349.pub4. PMC 6485957. PMID 29156493.
- ↑ Thieme H, Mehrholz J, Pohl M, Behrens J, Dohle C (January 2013). "Mirror therapy for improving motor function after stroke". Stroke. 44 (1): e1-2. doi:10.1161/strokeaha.112.673087. PMID 23390640.
- ↑ Fregni F, Pascual-Leone A (July 2007). "Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS". Nature Clinical Practice. Neurology. 3 (7): 383–93. doi:10.1038/ncpneuro0530. PMID 17611487.
- ↑ Balasubramanian S, Klein J, Burdet E (December 2010). "Robot-assisted rehabilitation of hand function". Current Opinion in Neurology. 23 (6): 661–70. doi:10.1097/WCO.0b013e32833e99a4. PMID 20852421.
- ↑ Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, van Wijck F (November 2014). Cochrane Stroke Group (ed.). "Interventions for improving upper limb function after stroke". The Cochrane Database of Systematic Reviews (11): CD010820. doi:10.1002/14651858.CD010820.pub2. PMC 6469541. PMID 25387001.
- ↑ Fryer CE, Luker JA, McDonnell MN, Hillier SL (August 2016). "Self management programmes for quality of life in people with stroke". The Cochrane Database of Systematic Reviews. Art. No.: CD010442 (8): CD010442. doi:10.1002/14651858.CD010442.pub2. PMC 6450423. PMID 27545611.
- ↑ 194.0 194.1 Coffey, C. Edward; Cummings, Jeffrey L; Starkstein, Sergio; Robinson, Robert (2000). Stroke – the American Psychiatric Press Textbook of Geriatric Neuropsychiatry (Second ed.). Washington DC: American Psychiatric Press. pp. 601–17.
- ↑ Stanford Hospital & Clinics. "Cardiovascular Diseases: Effects of Stroke". Archived from the original on 2009-02-10.
- ↑ Reith J, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS (August 1997). "Seizures in acute stroke: predictors and prognostic significance. The Copenhagen Stroke Study". Stroke. 28 (8): 1585–9. doi:10.1161/01.STR.28.8.1585. PMID 9259753. Archived from the original on 2013-01-12.
- ↑ Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C (December 1997). "Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project". BMJ. 315 (7122): 1582–7. doi:10.1136/bmj.315.7122.1582. PMC 2127973. PMID 9437276.
- ↑ editors, Tom A. Schweizer, R. Loch Macdonald (2014). The behavioral consequences of stroke. New York [u.a.]: Springer. pp. 119–33. ISBN 978-1-4614-7671-9.
- ↑ Mackenzie, Catherine (2011). "Dysarthria in stroke: A narrative review of its description and the outcome of intervention". International Journal of Speech-Language Pathology. 13 (2): 125–36. doi:10.3109/17549507.2011.524940. PMID 21480809. Archived from the original on 2020-08-07. Retrieved 2020-07-19.
- ↑ Ackley, Betty; Ladwig, Gail B.; Kelley, Helen (2010). Nursing diagnosis handbook: an evidence-based guide to planning care (9th ed.). Maryland Heights, MO.: Mosby.
- ↑ 201.0 201.1 Senelick Richard C., Rossi, Peter W., Dougherty, Karla (1994). Living with Stroke: A Guide for Families. Contemporary Books, Chicago. ISBN 978-0-8092-2607-8. OCLC 40856888.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Allida, Sabine; Cox, Katherine Laura; Hsieh, Cheng-Fang; House, Allan; Hackett, Maree L. (11 May 2020). "Pharmacological, psychological and non-invasive brain stimulation interventions for preventing depression after stroke". The Cochrane Database of Systematic Reviews. 5: CD003689. doi:10.1002/14651858.CD003689.pub4. ISSN 1469-493X. PMC 7211517. PMID 32390167. Archived from the original on 29 August 2021. Retrieved 19 July 2020.
- ↑ 203.0 203.1 Allida, Sabine; Cox, Katherine Laura; Hsieh, Cheng-Fang; Lang, Helen; House, Allan; Hackett, Maree L. (28 January 2020). "Pharmacological, psychological, and non-invasive brain stimulation interventions for treating depression after stroke". The Cochrane Database of Systematic Reviews. 1: CD003437. doi:10.1002/14651858.CD003437.pub4. ISSN 1469-493X. PMC 6999797. PMID 31989584.
- ↑ 204.0 204.1 Villarosa, Linda; Singleton, Lafayette; Johnson, Kirk A. (1993). The Black health library guide to stroke. New York: Henry Holt and Co. ISBN 978-0-8050-2289-6. OCLC 26929500.
- ↑ Leigh R, Oishi K, Hsu J, Lindquist M, Gottesman RF, Jarso S, et al. (August 2013). "Acute lesions that impair affective empathy". Brain. 136 (Pt 8): 2539–49. doi:10.1093/brain/awt177. PMC 3722353. PMID 23824490.
- ↑ Hamilton RH, Chrysikou EG, Coslett B (July 2011). "Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation". Brain and Language. 118 (1–2): 40–50. doi:10.1016/j.bandl.2011.02.005. PMC 3109088. PMID 21459427.
- ↑ Leys D, Hénon H, Mackowiak-Cordoliani MA, Pasquier F (November 2005). "Poststroke dementia". The Lancet. Neurology. 4 (11): 752–9. doi:10.1016/S1474-4422(05)70221-0. PMID 16239182.
- ↑ Kuźma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ (November 2018). "Stroke and dementia risk: A systematic review and meta-analysis". Alzheimer's & Dementia. 14 (11): 1416–1426. doi:10.1016/j.jalz.2018.06.3061. PMC 6231970. PMID 30177276.
- ↑ Coulthard E, Singh-Curry V, Husain M (December 2006). "Treatment of attention deficits in neurological disorders". Current Opinion in Neurology. 19 (6): 613–8. doi:10.1097/01.wco.0000247605.57567.9a. PMID 17102702.
- ↑ Lim C, Alexander MP (December 2009). "Stroke and episodic memory disorders". Neuropsychologia. 47 (14): 3045–58. doi:10.1016/j.neuropsychologia.2009.08.002. PMID 19666037.
- ↑ Murray ED, Buttner N, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds.). Bradley's neurology in clinical practice. Vol. 1 (6th ed.). Philadelphia: Elsevier/Saunders. pp. 100–01. ISBN 978-1-4377-0434-1.
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 11 November 2009. Retrieved November 11, 2009.
- ↑ "The top 10 causes of death". WHO. Archived from the original on 2013-12-02.
- ↑ "Why South Asians Facts". Indian Heart Association. Archived from the original on May 18, 2015. Retrieved May 8, 2015.
- ↑ Towfighi A, Saver JL (August 2011). "Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead". Stroke. 42 (8): 2351–5. doi:10.1161/STROKEAHA.111.621904. PMID 21778445.
- ↑ Ellekjaer H, Holmen J, Indredavik B, Terent A (November 1997). "Epidemiology of stroke in Innherred, Norway, 1994 to 1996. Incidence and 30-day case-fatality rate". Stroke. 28 (11): 2180–4. doi:10.1161/01.STR.28.11.2180. PMID 9368561. Archived from the original on February 28, 2008.
- ↑ Bongers TN, de Maat MP, van Goor ML, Bhagwanbali V, van Vliet HH, Gómez García EB, et al. (November 2006). "High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability". Stroke. 37 (11): 2672–7. doi:10.1161/01.STR.0000244767.39962.f7. PMID 16990571.
- ↑ Ashrafian H (April 2010). "Familial stroke 2700 years ago". Stroke. 41 (4): e187, author reply e188. doi:10.1161/STROKEAHA.109.573170. PMID 20185778.
- ↑ 219.0 219.1 Thompson JE (August 1996). "The evolution of surgery for the treatment and prevention of stroke. The Willis Lecture". Stroke. 27 (8): 1427–34. doi:10.1161/01.STR.27.8.1427. PMID 8711815.
- ↑ Kopito, Jeff (September 2001). "A Stroke in Time". MERGINET.com. 6 (9). Archived from the original on 2012-12-08.
- ↑ R. Barnhart, ed. The Barnhart Concise Dictionary of Etymology (1995)
- ↑ Schiller F (April 1970). "Concepts of stroke before and after Virchow". Medical History. 14 (2): 115–31. doi:10.1017/S0025727300015325. PMC 1034034. PMID 4914683.
- ↑ Finger, Stanley; Boller, François; Tyler, Kenneth L. (2010). Handbook of Clinical Neurology. North-Holland Publishing Company. p. 401. ISBN 978-0-444-52009-8. Archived from the original on 12 October 2013. Retrieved 1 October 2013.
- ↑ Scadding, John W. (2011). Clinical Neurology. CRC Press. p. 488. ISBN 978-0-340-99070-4. Archived from the original on 12 October 2013. Retrieved 1 October 2013.
- ↑ Sirven, Joseph I.; Malamut, Barbara L. (2008). Clinical Neurology of the Older Adult. Lippincott Williams & Wilkins. p. 243. ISBN 978-0-7817-6947-1. Archived from the original on 12 October 2013. Retrieved 1 October 2013.
- ↑ Kaufman, David Myland; Milstein, Mark J (5 December 2012). Kaufman's Clinical Neurology for Psychiatrists. Elsevier Health Sciences. p. 892. ISBN 978-1-4557-4004-8. Archived from the original on 12 October 2013. Retrieved 1 October 2013.
- ↑ 227.0 227.1 Mosby's Medical Dictionary, 8th edition. Elsevier. 2009.
- ↑ "What is a Stroke/Brain Attack?" (PDF). National Stroke Association. Archived (PDF) from the original on 19 October 2013. Retrieved 27 February 2014.
- ↑ Segen's Medical Dictionary. Farlex, Inc. 2010.
- ↑ Morris, Dylan R.; Ayabe, Kengo; Inoue, Takashi; Sakai, Nobuyuki; Bulbulia, Richard; Halliday, Alison; Goto, Shinya (1 March 2017). "Evidence-Based Carotid Interventions for Stroke Prevention: State-of-the-art Review". Journal of Atherosclerosis and Thrombosis. 24 (4): 373–387. doi:10.5551/jat.38745. ISSN 1340-3478. PMC 5392474. PMID 28260723.
Further reading
- Mohr JP, Choi D, Grotta J, Wolf P (2004). Stroke: Pathophysiology, Diagnosis, and Management. New York: Churchill Livingstone. ISBN 978-0-443-06600-9. OCLC 50477349.
- Warlow CP, van Gijn J, Dennis MS, Wardlaw JM, Bamford JM, Hankey GJ, Sandercock PA, Rinkel G, Langhorne P, Sudlow C, Rothwell P (2008). Stroke: Practical Management (3rd ed.). Wiley-Blackwell. ISBN 978-1-4051-2766-0.
External links
- Stroke at Curlie
- DRAGON Score for Post-Thrombolysis Archived 2020-10-27 at the Wayback Machine
- THRIVE score for stroke outcome Archived 2016-09-13 at the Wayback Machine
- National Institute of Neurological Disorders and Stroke Archived 2020-07-23 at the Wayback Machine
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- Pages containing links to subscription-only content
- CS1: long volume value
- All articles with dead external links
- Articles with dead external links from September 2018
- Articles with invalid date parameter in template
- Articles with permanently dead external links
- Webarchive template wayback links
- CS1 maint: multiple names: authors list
- Articles with hatnote templates targeting a nonexistent page
- All articles with unsourced statements
- Articles with unsourced statements from June 2016
- Articles with unsourced statements from January 2017
- Articles with unsourced statements from October 2012
- Articles with unsourced statements from October 2016
- Articles with unsourced statements from August 2020
- Articles with Curlie links
- Stroke
- Causes of death
- RTT
- RTTEM